|
* This work was supported in part by NIH Grants
CA 28740, CA 24369, and CA 18862. J. Ritz is a Special Fellow of
the Leukemia Society of America.
R. C. Bast is a Scholar of the Leukemia Society of America. J. M.
Lipton is a Dyson Foundation Investigator in Pediatric Oncology
A. Introduction
Monoclonal antibodies which are specific for surface antigens of
leukemic cells have become useful diagnostic reagents and have been
used to dissect the heterogeneity of leukemia in man [ I, 2]. In
addition, it is apparent that large quantities of homogeneous antibody
which primarily react with leukemic cells may become useful therapeutic
reagents. Previous trials of serotherapy with various monoclonal
antibodies in patients with multiply relapsed acute lymphoblastic
leukemia (ALL) or lymphoma have demonstrated that intravenously
administered antibody can rapidly bind to tumor cells in peripheral
blood and bone marrow and that relatively large numbers of malignant
cells can be eliminated in vivo [3-6]. In one patient with B-cell
lymphoma, a cQmplete remission was achieved following intravenous
infusion of monoclonal anti-idiotype antibody [7]. In general, however,
these studies have not produced clinically significant responses
and have clearly identified several specific factors such as presence
of serumblocking factors, antigenic modulation, and inefficiency
of natural effector mechanisms, which limit the therapeutic activity
of monoclonal antibody in vivo (reviewed in[8]). One approach which
circumvents several of the obstacles to effective serotherapy in
vivo is the utilization of monoclonal antibody in vitro. Thus, in
a controlled in vitro environment, extracellular blocking factors
can be removed, incubation with monoclonal antibody at 4 °C can
effectively inhibit antigenic modulation, and multiple treatments
with heterologous complement can be used to ensure the lysis of
all tumor cells. In addition, potential cross reactivity of monoclonal
antibodies with nonhematopoietic tissues can be avoided. A previous
report has presented our preliminary experience with the use of
the J5 monoclonal antibody and rabbit complement to treat bone marrow
in vitro to remove residual leukemic cells prior to autologous transplantation
[9]. This report summarizes the current results of this clinical
study.
B. Methods
I. J5 Monoclonal Antibody
The method for generation and characterization of J5 monoclonal
antibody specific for the common acute lymphoblastic leukemia antigen
(CALLA) has been described previously [ 10]. ]5 antibody (murine
IgG2A) is reactive with leukemic cells from 80% of patients with
non- T cell ALL and 40% of patients with chronic myelocytic leukemia
in blast crisis. In addition, lymphoma cells from almost all patients
with B-cell nodular poorly differentiated lymphocytic lymphoma and
Burkitt's lymphoma, and 45% of patients with T -cell lymphoblastic
lymphoma, are reactive with 15 antibody [I I]. Within normal bone
marrow, approximately 1% of cells also express CALLA, but previous
studies have shown that these normal CALLA-positive cells are not
myeloid precursor cells (CFU-C, BFUE, CFU-E, and CFU-G/E) [12].
More recent studies have indicated that CALLA is expressed during
early lymphoid cell differentiation, but it appears that the earliest
lymphoid stem cells do not express this antigen [13]. In addition
to hematopoietic cells, it has been demonstrated that 15 antibody
is reactive with various nonhematopoietic tissues including cells
from renal glomerulus and proximal tubules [14]. Recently, it has
also been found that 15 antibody is reactive with cultured fibroblasts
from normal bone marrow (1. Ritz, unpublished observation) as well
as cell lines established from various solid tumors (H. Lazarus,
personal communication). These findings are of particular importance
for the therapeutic application of CALLA-specific antibodies since
these normal cells would also be potential targets for antibody-directed
therapy. Our method for obtaining large quantities of purified 15
monoclonal antibody and our method for in vitro treatment of bone
marrow have been previously described [9, 15, 16]. Briefly, 15 antibody
was obtained aseptically from ascitic fluid of Balb/c mice that
had been primed with pristane followed by intraperitoneal inoculation
of 15 hybridoma cells. Bone marrow was harvested from anterior and
posterior iliac crests under general anesthesia, and mononuclear
cells were isolated using discontinuous Ficoll-Hypaque density gradients.
Bone marrow cells were then treated three times with 15 antibody
and rabbit complement and cryopreserved in the vapor phase of liquid
nitrogen in media containing 10% DMSO and 90% autologous seurm.
Prior to infusion, cryopreserved marrow was rapidly thawed and cells
were diluted in medium which contained DNAase to prevent clumping.
C. Results
I. Clinical Protocol
All patients with ALL who had relapsed following standard chemotherapy
and whose leukemic cells expressed the com mon ALL antigen (CALLA)
were considered eligible for the protocol that is outlined in Fig.
I. Patients who had normal identical twins or histocompatible siblings
were ineligible for this study and received either syngeneic or
allogeneic bone marrow transplantation. In addition, patients in
whom a complete remission could not be induced with chemotherapy
alone were excluded. Following induction of second or subsequent
remission, patients received intensive chemotherapy with the following
agents: VM-26, cytosine arabinoside (araC), and L-asparaginase (Fig.
I ). CNS repropylaxis with intrathecal araC and hydrocortisone was
also adminstered at that time. After recovery from in tensification,
patients underwent bone marrow harvest under general anesthesia.
Mononuclear cells were isolated and treated three times with 15
antibody and rabbit complement prior to cryopreservation. A separate
aliquot of marrow was also cryopreserved without antibody treatment.
These cells constituted a "back-up marrow" which could be used in
the event that antibodytreated marrow failed to engraft but was
not used in any of our patients. In patients I, 2, and 3, "back-up
marrow" was harvested separately just prior to intensification and
cryopreserved without antibody treatment. One day after marrow harvest,
patients began receiving ablative treatment consisting of VM-26,
araC, cyclophosphamide, and total body irradiation (TBI) (Fig. I).
Approximately 12 h after TBI, cryopreserved marrow which had previously
been treated in vitro was rapidly thawed and reinfused through a
central venous catheter. Patients did not receive any additional
chemotherapy. Thus far, six patients have been treated under this
protocol and have been followed for more than 4 months. The clinical
history of these patients and their current status is summarized
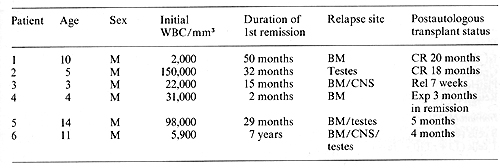
in Table I. Patient I had relapsed in bone marrow 20 months after
elective cessation of therapy and now continues in unmaintained
remission 20 months after autologous transplantation. Patient 2
had relapsed in both testes 2 months after completion of chemotherapy.
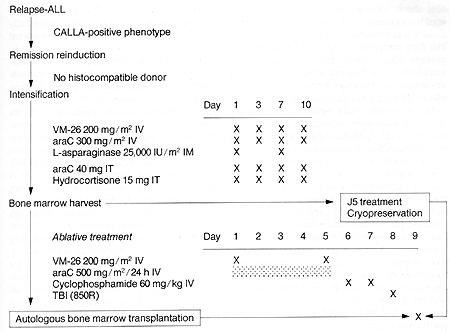
Fig. I. Clinical protocol for autologous transplantation
with ]5 antibody and complement-treated bone marrow
Bone marrow at that time contained 7% Iymphoblasts.
He continues in unmaintained remission 18 months after autologous
bone marrow transplantation. Patient 3 first relapsed in the CNS
while receiving systemic chemotherapy and later relapsed in the
bone marrow as well. A second bone marrow remission was difficult
to achieve and was only attained after 4 months of intensive chemotherapy.
He relapsed with CALLA-positive lymph ob lasts 7 weeks after transplantation.
Patient 4 relapsed in the bone marrow 3 months after initial diagnosis.
He tolerated the ablative regimen well but subsequently developed
interstitial pneumonitis, which was probably secondary to cytomegalovirus
infection and expired 3 months after transplantation. Pneumonitis
was also complicated by intrapulmonary hemorrhage secondary to persitent
thrombocytopenia. At autopsy, there was no evidence of leukemic
relapse. Patient 5 was transplanted in third remission. He first
relapsed in the bone marrow 30 months after initial diagnosis. and
subsequently continued on chemotherapy for an additional 4 years
until therapy was electively stopped. Testicular relapse with CALLA-positive
cells occurred 8 months later. Morphologic examination of bone marrow
at this time demonstrated 3% blasts but immunofluorescence analysis
of purified mononuclear cells demonstrated 19% CALLA-positive cells.
He was subsequently entered onto our protocol and continues in remission
5 months after transplantation. Patient 6 received chemotherapy
for 5 years after initial diagnosis but relapsed simultaneously
in the bone marrow, CNS, and testes 2 years after elective cessation
of therapy. Following reinduction of a second complete remission,
he received the intensification and ablative therapy outlined in
Fig. I. He continues in remission 4 months after autologous trans
plantation.
Table I. Clinical characteristics of patients treated
with autologous bone marrow transplantation
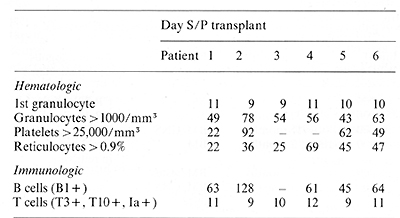
II. Hematopoietic Reconstitution
Hematopoietic engraftment in six patients following autologous
transplantation with J5-treated bone marrow is summarized in Table
2. In patient I, the first evidence of marrow engraftment was seen
II days after marrow infusion, and subsequent recovery of granulocytes,
reticulocytes, and platelets occurred promptly. In patient 2, the
first evidence of marrow engraftment was seen 9 days after transplant,
but subsequent he matopoietic recovery occurred slowly. Although
complete recovery did eventually occur, severe thrombocytopenia
persisted for 3 months. In patient 3, hematopoietic reconstitution
proceeded gradually after marrow infusion, but bone marrow relapse
became evident before complete recovery of peripheral counts had
occurred. Seven weeks post transplant, bone marrow aspirate demonstrated
engraftment of granulocytic, erythroid, and megakaryocytic precursors
but also contained approximately 40% CALLA-positive Iymphoblasts.
Patient 4 exhibited prompt recovery of granulocytes but reconstitution
of both platelets and reticulocytes was much slower. Although megakryocytes
were present in bone marrow aspirates and at autopsy, circulating
platelet counts remained < 20,000 mm³, Patients 5 and 6 have been
followed for relatively short periods, but hematopoietic recovery
in both of these patients appears to be comparable to that seen
in previous patients.
III. Immunologic Reconstitution
The appearance of B cells in peripheral blood and bone marrow was
detected by reactivity with monoclonal antibody B I which identifies
a unique antigen expressed by normal B cells [17]. In patients I
and 2, serum immunoglobulin levels gradually increased following
the appearance of B 1positive cells. In all six patients, T -lymphocytes
were the first cells to engraft following transplantation. These
cells expressed T3, TIO [18], and la antigens [ 19]. Although T
cells from
Table 2. Hematopoietie recovery following
autologous bone marrow transplantation
peripheral blood normally express T3 antigen, both TIO and la antigens
are normally expressed only after cell activation [20, 21 ]. Both
T4 cells (T -inducer phenotype) and T8 cells (T -suppressor phenotype)
were present, but the relative percentage of these cells in peripheral
blood varied during engraftment. In almost all patients, the T4/T8
ratio of circulating T cells was abnormally low. In patients who
initially had normal percentages of T4-positive cells (patients
I and 5), peripheral T cells also later became predominantly T8
positive. The number of T cells which were la positive gradually
decreased during the first 4 months following engraftment. Expression
of T 10 antigen also gradually decreased following engraftment but
persisted much longer. At no time during engraftment was T6 antigen
expressed by peripheral blood cells.
D. Discussion
Autologous bone marrow transplantation has previously been used
in patients with various malignant diseases in an effort to circumvent
marrow toxicity and to allow the administration of otherwise lethal
doses of chemotherapy. Unfortunately, in most patients with solid
tumors, higher doses of chemotherapy have not resulted in more effective
eradication of malignant cells. In contrast, it has been demonstrated
that leukemia and lymphoma cells can be eradicated with intensive
chemotherapy and total body irradiation (TBI) even when these tumors
are resistant to conventional doses of chemotherapy. This has led
to the successful treatment of acute leukemia with ablative chemotherapy
and TBI in conjunction with bone marrow transplantation from identical
twins or allogeneic histocompatible siblings [22-25]. Unfortunately,
the utilization of ablative therapy in leukemia is restricted to
approximately 40% of patients who have normal histocompatible marrow
donors, and autologous marrow transplantation is limited by the
fact that residual leukemia is present in the patient's marrow,
even during complete remission, Previous studies have attempted
to circumvent this problem through the use of physical separation
techniques [26] or treatment with conventional rabbit heteroantisera
[27, 28] to eliminate leukemic cells prior to autologous transplantation.
Hightiter monoclonal antibodies which activate complement and specifically
react with leukemic cells and not with hematopoietic stem cells
are potentially very useful reagents which can be used to eliminate
small numbers of leukemic cells in the presence of a large excess
of normal marrow. The utilization of these reagents in vitro may
therefore allow the application of autologous bone marrow transplantation
to patients who do not otherwise have histocompatible donors. In
the present study, six patients with relapsed ALL received ablative
therapy with VM-26, araC, cyclophosphamide, and TBI followed by
infusion of autologous remission bone marrow which had been treated
in vitro with J5 antibody and rabbit complement to remove residual
leukemic cells. Hematopoietic engraftment with ]5-treated bone marrow
occurred in all six patients. Reconstitution of B cells and immunoglobulin
production occurred after reconstitution of myeloid cells. Since
all of our patients have engrafted with J5 antibodytreated bone
marrow and two patients have been in unmaintained remission for
more than 18 months, our study suggests that this approach may be
a feasible alternative to conventional chemotherapy in patients
with relapsed ALL. Treatment of additional patients and longer fallow-up
periods will be necessary to determine if in vitro antibody treatment
is a clinically effective therapeutic modality.
References
1. Nadler LM, Ritz J, Griffin JD, Todd RF, Reinherz EL, Schlossman
SF (1981) Diagnosis and treatment of human leukemias utilizing monoclonal
antibodies. Prog Hematol12: 187-226
2. Greaves MF, Robinson JB, Delia D, Ritz J, Schlossman SF, Sieff
C, Goldstein G, Kung PC, Bollum F, Edwards P (1981) Comparative
antigenic phenotypes of normal and leukemia hematopoietic precursor
cells anaIyzed with a "library" of monoclonal antibodies. In: Neth
R, Gallo RC, Graf T, Mannweiler K, Winkler K (eds) Modern Trends
in Human Leukemia IV. SpringerVerlag, Berlin Heidelberg New York
pp 296-304
3. Nadler LM, Stashenko P, Hardy R, Kaplan WD, Button LN, Kufe DW,
Antman KH, Schlossman SF (1980) Serotherapy of a patient with a
monoclonal antibody directed against a human lymphoma associated
anti gen. Cancer Res40:3147-3154
4. Ritz J, Pesando JM, Sallan SE, Clavell LA, Notis-McConarty J,
Rosenthal P, Schlossman SF (1981) Serotherapy of acute lymphoblastic
leukemia with monoclonal antibody. Blood 58: 141-152
5. Miller RA, Maloney DG, Mc Killop J, Levy R (1981) In vivo effects
of murine hybridoma monoclonal antibody in a patient with T -cellleukemia.
Blood 58: 78-86
6. Miller RA, Levy R ( 1981) Response of cutaneous T -cell lymphoma
to therapy with hybridoma monoclonal antibody. Lancet II: 226-230
7. Miller RA, Maloney DA, Warnke R, Levy R ( 1982) Treatment of
B-cell lymphoma with monoclonal anti-idiotype antibody. N Engl J
Med306:517-522
8. Ritz J, Schlossman SF ( 1982) Utilization of monoclonal antibodies
in the treatment of leukemia and lymphoma. Blood 59: I-II
9. Ritz J, Sallan SE, Bast RC, Lipton JM, Clavell LA, Feeney M,
Hercend T, Nathan DG, Schlossman SF ( 1982) Autologous bone marrow
transplantation in CALLA positive acute lymphoblastic leukemia after
in vitro treatment with J5 monoclonal antibody and complement. Lancet
11:60-63
10. Ritz J, Pesando JM, Notis-McConarty J, Lazarus H, Schlossman
SF ( 1980) A monoclonal antibody to human acute lymphoblastic leukemia
antigen. Nature 283: 583-585
II. Ritz J, Nadler LM, Bhan AK, Notis-McConarty J, Pesando JM, Schlossman
SF (1981) Expression of common acute lymphoblastic leukemia antigen
(CALLA) by lymphomas of B-cell and T -cell lineage. Blood 58:648-652
12. Clavell LA, Lipton JM, Bast RC, Kudisch M, Pesando JM, Schlossman
SF, Ritz J (1981) Absence of common ALL antigen on bi-potent myeloid,
erythroid and granulocyte progenitors. Blood 58: 333-336
13. Hokland P, Rosenthal P, Griffin JD, Nadler LM, Daley JF, Hokland
M, Schlossman SF, Ritz J ( 1983) Purification and characterization
of fetal hematopoietic cells which express the common acute lymphoblastic
leukemia antigen (CALLA) J Exp Med (in press)
14. Metzgar RS, Borowitz MJ, Jones NH, Dowell BL (1981) Distribution
of common acute lymphoblastic leukemia antigen in non-hematopoietic
tissues. J Exp Med 154: 1249-1254
15. Feeney M, Knapp RC, Greenberger JS, Bast RC ( 1981) Elimination
of leukemic cells from rat bone marrow using antibody and complement.
Cancer Res 41: 3331-3335
16. Bast RC, Ritz J, Lipton JM, Feeney M, Sal Ian SE, Nathan DG,
Schlossman SF ( 1983) Elimination of leukemic cells from human bone
marrow using monoclonal antibody and complement Cancer Res (in press)
17. Stashenko P, Nadler LM, Hardy R, Schlossman SF ( 1980) Characterization
of a human B lymphocyte specific antigen. J Immunol 125:1678-1685
18. Reinherz EL, Schlossman SF ( 1980) The differentiation and function
of human T lymphocytes: A review. Cell 19: 821-827
19. Nadler LM, Stashenko P, Hardy R, Pesando JM, Yunis EJ, Schlossman
SF (1981) Monoclonal antibodies defining serologically distinct
HLA-D/DR related la-Iike antigens in man. Human Immunoll:77-90
20. Reinherz EL, Kung PC, Pesando JM, Ritz J, Goldstein G, Schlossman
SF (1979) la determinants on human T -cell subsets defined by monoclonal
antibody: Activation stimuli required for expression. J Exp Med
150:1472-1482
21. Hercend T, Ritz J, Schlossman SF, Reinherz EL (1981) Comparative
expression of T9. TlO and la antigens on activated human T -cell
subsets. Human Immunol 3:247-259
22. Fefer A, Cheever MA, Thomas ED, Appelbaum FR, Buckner CD, Clift
RA, Glucksberg H, Greenberg PD, Johnson FL Kaplan HG, Sanders JE,
Storb R, Weiden PL ( 1981) Bone marrow transplantation for refractory
acute leukemia in 34 patients with identical twins. Blood 57:421-430
23. Johnson FL Thomas ED, Clark BS, Chard RL Harmann JR, Storb R
(1981) A comparison of marrow transplantation with chemotherapy
for children with acute lymphoblastic leukemia in second or subsequent
remission. N Engl J Med 305: 846-851
24. Blume KG, Beutler E, Bross KJ, Chillar RK, Ellington OB, Fahey
JL Farbstein MJ, Forman SJ, Schmidt GM, Scott EP, Spruce WE, Turner
MA, Wolf JL (1980) Bone marrow ablation and allogeneic marrow transplantation
in acute leukemia. N Engl J Med 302:1041-1046
25. Clift RA, Buckner D, Thomas ED, Sanders JE, Stewart PS, McGuffin
R, Hersman J, Sullivan KM, Sale GE, Storb R (1982) Allogeneic marrow
transplantation for acute lymphoblastic leukemia in remission using
fractionated total body irradiation. Leuk Res 6:409-412
26. Dickie KA, McCredie KB, Spitzer G, Zan der A, Peters L Verma
DS, Stewart D, Keating M, Stevens EE (1978) Autologous bone marrow
transplantation in patients with adult acute leukemia in relapse.
Transplant. 26: 169-173
27. Wells JR, Billing R, Herzog P, Feig SA, Gale RP, Terasaki P,
Cline MJ (1979) Autotransplantation after in vitro im munotherapy
of lymphoblastic leukemia. Exp Hematol7 (suppl) 5: 164-169
28. Netzel B, Rodt H, Haas RJ, Kolb HJ, Thierfelder S (1980) Immunologic
conditioning of bone marrow for autotransplantation in childhood
acute Iym phoblastic leukemia Lancet 1: 1330-1332
|