|
1 Department of Molecular Biology. University of
Brussels. 67, rue des Chevaux. 1640 Rhode St-Genese. Belgium
2 Faculty of Agronomy. 5800 Gembloux, Belgium
3 Pasteur Institute. rue du Remorqueur, 1140 Bruxelles. and Vrije
Universiteit Brussel. 1050 Brüssel. Belgium
4 National Institute for Veterinary Research. 99. Groeselenberg.
1180 Uccle. Bruxelles. Belgium
Abbreviations Used
ID gp 60: Immunodiffusion test based on BLV glycoprotein (MW =
60000) as antigen
RIA gp 60: Radioimmunoassay test based on BLV glycoprotein (MW =
60000) as antigen
RIA p 24: Radioimmunoassay test based on BLVinternal protein p 24
(MW= 24000) as antigen
BL V: Bovine Leukemia Virus
PL: Persistent Lymphocytosis
EBL: Enzootic Bovine Leucosis
SBL: Sporadic Bovine Leucosis
I. Enzootic Bovine Leucosis: The Disease
One generally distinguishes two types of bovine leucoses: an enzootic
type and a sporadic type [3]. Here. we will be solely dealing with
the enzootic type, the so-called Enzootic Bovine Leucosis (EBL)
The basic features of this lymphoproliferative disease are:
- it is contagious; it spreads within a herd through contacts, saliva,
milk, ... and from herd to herd mainly through commercial exchanges;
- it is induced by Bovine Leukemia Virus (BLV) a retrovirus exogenous
to the bovine species [10];
-it involves the B lymphocytes [15, 17]
-it can be easily transmitted by the virus to cattle or sheep. Experimental
BL V infection (but no clinical disease, sofar) has been obtained
in goats and chimpanzees. No natural transmission of BL V to man
seems to occur [3.6];
- BL V infected animals develop a humoral response directed against
the viral antigens.
Enzootic bovine leucosis is a chronic disease that develops over
along period of time (several years generally). In natural conditions,
very few cattle less than 2 years of age harbor antibodies to BLVantigens
[11]. The same is true where BLVis searched for by its biological
property of inducing syncytia [7] or early polykaryocytosis. If,
however, a search is made among the off spring of BL V infectedparents,
it appears that as much as 14% of calves are infected at birth.
As discussed in [3], this situation reflects congenital infection
by BL V and not true vertical transmission. BL V infection always
induces a humoral antibody response and sometimes induces an hematological
disorder called "Persistent Lymphocytosis" (PL). In such cases,
the lymphocyte population is made of normal cells and a variable
percentage of tumor cells as proven by molecular hybridization studies
[3, 10]. PL has a genetic background [I] being much more frequent
in some families within a breed than in other families of the same
breed. With time, tumor development may occur, a phenomenon very
poorly understood at the present time. Tumors may appear practically
everywhere, in the digestive tract, the respiratory tract, muscles,
...but they are always lymphoid. Most lymph nodes are enlarged,
sometimes some of them only [2, 14,19].
2. BL V: The Causative Agent of EBL
BLV is a retrovirus [3] produced in large quantities by essentially
two cell culture systems, the Fetal Lamb Kidney cell-line [18] and
the TblLu, a bat cell-line [13]. Morphologically the virus can be
considered as a C-type although it displays some unusual peculiarities
[3].
2.1. BLV Genome
It is a 60-70S RNA molecule associated with reverse transcriptase.
The number of genes and their order along the RNA molecule are not
precisely known so far. DNA complementary to the RNA genome and
representative of it, has been synthesized and extensively used
in molecular hybridization experiments. The results of these studies
are illustrated in Fig. I A and 1 Band call for the following comments:
If we take salmon sperm DNA as a control (histogram II) it appears
that normal bovine DNA hybridizes some 4% better than the control.
We now know that this is due to contamination of 70S RNA used as
template by 28S ribosomal RNA. DNAs from FLK-BLV producing cells
(histogram 2). from buffy coat cells of an animal in persistent
lymphocytosis with tumor (histogram 4) and from bovine enzootic
tumor (histogram 5) hybridize with a maximum of 45% of the probe
at a Cot value of30000. This result is compatible with one proviral
DNA copy per haploid genome, if every cell contains the viral information.
DNAs from tissues infiltrated with tumorous lymphocytes, hybridize
to BLVc DNA to an extent that is roughly proportional to the degree
of infiltration (histograms 3.6 and 7). Sheep infected by BLV (histograms
9 and 10) show the same pattern ofhybridization as cattle do. DNAs
from human leukemic cells do not anneal to BLVc DNA.
That BL V is exogenous to the bovine genus was definitely established
by recycling the 3H BL V c DNA probe on normal bovine DNA. Results
are illustrated in Fig. I B. They prove 1°) that BL V is largely
if not totally exogenous to the bovine genus, 2°) that EBL is an
infections disease, thus amenable to eradication.
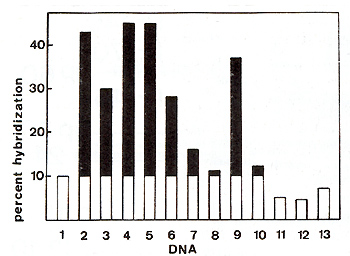
Fig. lA. Hybridization of HLV 3H cDNA to various bovine,
ovine and human cellular DNAs, Hybridizations between 2400 cpm of
³H cDNA (specific activity: 1,8 X 10 high 7 cpm/µg) and 250 µg of
cellular DNA were performed in 0,4 M phosphate buffer (pH = 6,8)
and 0,05% SDS in a final volume of 85 µI at 68°C. At a Cot value
of 30000, samples were assayed for S1 resistance, Source ofDNA:
1234567 DNA
1. Normal buffy coat cells.
2. FLK cell line. 3. Huffy coat cells from a cow in persistent lymphocytosis
without tumors.
4. Huffy coat cells from a cow in persistent lymphocytosis with
tumors.
5. EBL tumor.
6. Liver moderately infiltrated with lymphocytes (EBL).
7. Kidney slightly infiltrated with lymphocytes (EBL).
8. Tumorous lymph node from an SBL case.
9. Cutaneous tumor from a sheep infected with BLV.
10. Liver from the same leukemic sheep.
11. Salmon sperm.
12. Human chronic lymphatic leukemia. 13. Human chronic lymphatic
leukemia.
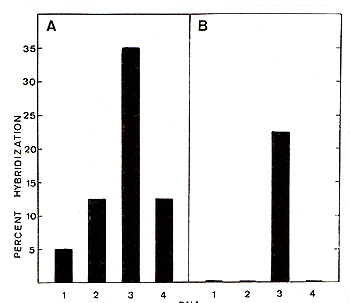
Fig. l B. Hybridization of BL V 3H cDNA (panel A) and
recycled BLV 3H cDNA (panel B) to the following cellular DNAs: 1.
Salmon sperm; 2. normal bovine buffy coat cells; 3. EBL tumor: 4.
SL tumorous lymph node. 2400 cpm of BLV ³H cDNA ( or recycled cDNA)
and 250 µg of cellular DNA were hybridized in 0,4 M phosphate buffer
(pH = 6,8) and 0,05% SDS. At a cellular Cot value of 30000 samples
were assayed tor S1 resistance
2.2. BLV Proteins
BLV virions, at least, contain: 2 glycoproteins: gp60 and gp30
linked together within the virus envelope 4 non-glycosylated polypeptides
p24, p 15, p 12 and p 10 one reverse transcriptase, MW= 58000-70000
([4] and Drescher et al., in prepara tion ) That the above mentioned
proteins are indeed viral antigens rests upon two lines of evidence
:
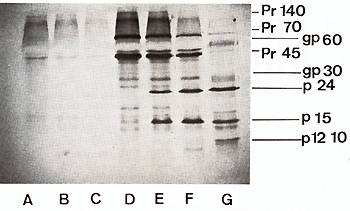
Fig.2. Fluorograph of SOS-polyacrylamide gel of immune
precipitates. Oocytes microinjected with a 1mg/ml solution of 30-40S
BLV RNA were labeled for 20 hours in Barth medium with 2 mg/ml of
³H leucine. lysed immediately (0) or chased in culture medium containing
excess unlabeled leucine for 100 hours (E) and 300 hours (F) and
then lysed. Non injected control oocytes were incubated in parallel
(A-C) and indirect immune precipitation carried out on the same
amount of homogenate with 4 µl of polyvalent anti-BLV serum.
After 2 hours at 37°C and overnight incubation at 4°C. 100 µl
of a 10% W/V Staphylococcus aureus suspension was added and incubation
continued for another 4 hours at 4°C. Bacterial suspension was collected
by centrifugation and washed. Immune complexes were separated by
boiling 2 minutes in SOS containing buffer and analyzed by electrophoresis
on a l5% polyacrylamide slab gel in the presence of SDS. G: ³H amino
acids labeled BLV marker
I. BLV infected animals synthesize antibodies directed against
at least 4 of them (gp60, gp30, p24, p 15).
2. BL V infected cells synthesize protein precursors to the gag
group (p 24, p 15). a presumed gag-pol precursor and a precursor
to BL V glycoproteins. In vitro protein synthesizing systems programmed
with BLV 30--40S viral RNA synthesize gag precursors and the putative
gag-pol precursor (Fig. 2). In adequate systems, these precursors
mature into viral structural antigens [8]. Subgenomic fractions
of BL V 35 S RNA code for a number of polypeptides with molecular
weights as 58000,45000,40000,35000, 18000, ...(Fig. 3). The 58000,45000
and 40000 Molecular weight polypeptides are coded by m RNAs sedimenting
in sucrose gradients in the 16 S to 18 S region and do not seem
to be related one to the other by fingerprint analysis. Apparently,
they are not of viral origin as preannealing of RNAs with BLV 35
S-c DNA does not block their synthesis in reticulocyte cell-free
systems. On the other hand, polypeptide 18000 is undoubtedly of
viral origin. Its biosynthesis in reticulocyte lysates is blocked
if 16S to 18S m RNA is preannealed with EL V 35 S-c DNA. Our present
efforts are attempting to identify the region of the BL V genome
coding for polypeptide 18000.
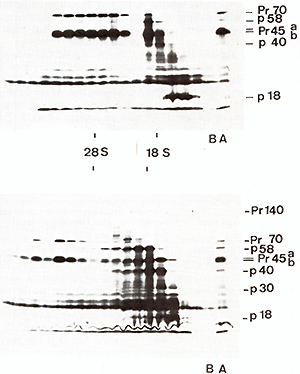
Fig.3. Fluorograph of SDS-polyacrylamide gel of translation
products of fractionated BLV virion RNA. Heat denaturated (95°C
for I minute in Tris-HC1. 10-² M, pH = 7.4. EDTA 10-³ M) BLV 60-70S
RNA (80 µg) was fractionated by oligo dT cellulose chromatography
and the polyA-containing fraction (lower panel) or poly-A-deficient
fraction (upper panel) were sedimented in a linear 15-30% glycerol
gradient in Tris-HCl 10-² M, pH = 7.4. NaCI 0.1 M. EDTA 0.0 1 M
in a SW 41 rotor at 40000 rpm for 4 hours at 20°C. The RNA of each
fraction was precipitated twice with ethanol, calf liver t-RNA being
added as a carrier. One fourth of the RNA of each fraction was used
to program protein synthesis in a messenger-dependent reticulocyte
cell-free lysate. Analysis of translation products is made on a
15% SDS-polyacrylamide slab gel. Track A: complete translation product
of poly A-containing (lower panel) or poly A-deficient (upper panel)
BLV RNA. Track B: control, no RNA added
3. Epidemiology of BLV
Search for an tibodies to BL V structural antigens is the present
basis of all epidemiological investigations and eradication campaigns
[3]. The presently most popular serological method is agar gel immunodiffusion
based on BLV gp60. but other techniques such as radioimmunoassays
([5] and Bex et al.. submitted), Early Polykaryocytosis Inhibition,
[9] VSV Pseudotypes Inhibition [20] are intensively investigated.
Direct assessment of EL V infection can even be obtained by Syncytia
or early polykaryocytosis induction but this is a too tedious process
for large scale application in field studies. In 1976-1977 [12]
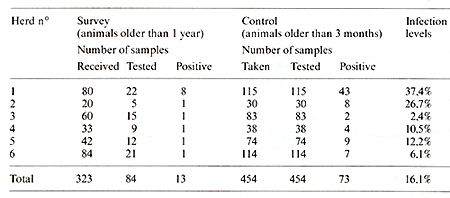
we applied JD gp60 in a survey of 1315 cattle herds (Table 1). In
a first round, we tested 1 blood sample out of 4. Every herd in
which one positive was detected was then more deeply investigated.
Every animal more than 3 months old was submitted to a blood test.
As can be seen in the last column of Table 1, the percentage of
infection observed by the method used. varied between 2,4 and 37,4%
with an average of 16,1%. For the whole study, 6 herds happened
to be infected by ELV over a total of 1315 examined, which leads
to an observed rate of infection of 0,46%. This number can be considered
as small in comparison with incidences reported for other countries
[3].
Table 1. Survey of 1315 herds by ID gp60 and Control
oft his survey
Within infected herds, we recently compared (Hex et al., submitted)
3 serological tests, namely ID gp60, RIA gp60 and RIA p24. Over
345 animals tested. 104 were positive in RIA gp60. 101 in ID gp60
and 99 in RIA p24. The investigated herds were most probably foci
of old EL V infections in which most "susceptible" animals had reached
such antibody levels that ID gp60 was almost as sensitive as RIA
gp60 and, indeed, more sensitive than RIA p 24. It should perhaps
be mentioned here that the Commission of European Communities recently
recommended to eradicate Enzootic Bovine Leucosis. The strict exogenous
character of ELV and its apparent low progression power make the
European recommendation quite feasible.
4. Host- Virus Relationship
The incoming of ELVinto a recipient immediately elicits an antibody
response to EL V structural antigens. The intensity of this response
probably depends on age of the host and its genetic make-up, virus
dose, health status, environment. ...In a recent study (Bex et al.,
submitted) we followed six sheep inoculated at birth, by the oral
route, by whole leukemic bovine blood. As a rule. antibody levels
to BL V gp 60 and p 24 rised steadily until the animal's death.
In parallel we followed the complement dependent cytotoxicity of
these sheep sera toward a BL V-producer cell line Fetal Lam b Kidney
cells [16]. Results examplified in Fig.4 clearly show that serum
cytotoxicity increased with time, reaching a maximum level in the
tumor phase of the disease at the animal's death. Immunoglobulins
active in the cytotoxic reaction belong to the Ig G1 sub-class.
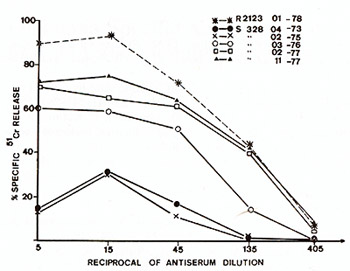
Fig.4. Evolution of cytotoxic activity of a sheep serum
(animal no 328) during the last two years of the animal's life.
Target cells were Fetal Lamb Kidney cells infected by and producing
BLV (These cells are most probably not transformed by BLV). One
of our best cytotoxic bovine sera ( R2123) was used here as a reference
5. Conclusions
The lymphoproliferative disease, Enzootic Bovine Leucosis, is an
infectious disease caused by a retrovirus called BLV (Bovine Leukemia
Virus). The nature and mode of action of virus gene products and
the mechanisms involved in the host-virus interplay are presently
under intense investigation.
Acknowledgements
We warmly thank the "Fonds Cancerologique de la Caisse Generale
d'Epargne et de Retraite". the European Economic Community (research
contract n° 00000150) and the Belgian Ministry of Agriculture for
their financial support. F.B. held a postdoctoral fellowship of
the "lnstitut pour l'Encouragement de la Recherche Scientifique
dans I'lndustrie et l'Agriculture". J.G. is "Assistant au Fonds
Cancerologique de la C.G.E.R.", R.K. and D.P. are "Aspirants du
Fonds National de la Recherche Scientifique". We greatly appreciated
the technical contributions of P. Ridremont. J. Severs. G. Vandendaele
and G. Vanheule.
References
1. Abt. D. A., Marshak. R. R.. Ferrer. J. F.. Piper. C. E.. Bhatt.
D. M.: Studies on the development of persistent lymphocytosis and
infection with the bovine C-type leukemia virus (BLV) in cattle.
Vet. Microbial. I, 287-300 (1976)
2. Anonymous: Criteria for the determination of the normal and leukotic
state in cattle. Prepared by an international committee on bovine
leukosis. J. Natl. Cancer Inst. 41,243-263 ( 1968)
3. Burny. A.. Bex. F.. Chantrenne. H.. Cleuter. Y.. Dekegel. D.,
Ghysdael. J.. Kettmann. R.. Leclercq. M.. Leunen. J.. Mammerickx.
M.. Portetelle. D.: Bovine leukemia virus involvement in enzootic
bovine leucosis. Ady. Cancer Res. 28,251-311 (1978)
4. Burny. A.. Bex. F.. Bruck. C.. Cleuter. Y.. Dekegel. D.. Ghysdael.
J.. Kettmann. R.. Leclercq. M.. Mammerickx. M.. Portetelle, D.:
Biochemical studies on enzootic and sporadic types of bovine leucosis.
In: Anti-viral mechanisms in thc control or neoplasia. Chandra,
P. (ed.). New York: Plenum Press 83-99 (1979)
5. Devare. S. G.. Stephenson. J. R.: Biochemical and immunological
characterization of the ma.jor envelope glycoprotein of bovine leukemia
virus. J. Viral. 23,443-447 ( 1977)
6. Donham. K.J.. van der Maaten. M.J.. Miller. J.M.. Kruse. B.C..
Rubino. M.J.: Seroeride miologic studies on the possible relationships
of human and bovine leukemia. .r. Natl. Cancer Lnst. 59,851-854
(1977)
7. Ferrer. J. F.. Piper. C. E., Baliga. V.: Diagnosis or BL v infection
in cattle of various ages. In: Bovine leucosis: Various methods
of molecular virology. Burny. A. (ed.). pr. 323-336. Luxembourg:
Commission of the European Communities 1977
8. Ghysdael. J.. Hubert. E.. Cleuter. Y.: Biosynthesis or bovine
leukemia virus major internal protein (p24) in in.jected cells and
xenopus laevis oocytes microin.jected with BLV 60-70S RNA. Arch.
internat. Physiol. Biochim. 85,978-979 ( 1977)
9. Guillemain. B.. Mamoun. R.. Levy. D.. Astier. T.. Parodi. A.
L.: Serological diagnosis of bovine leukemia virus infection by
early polykaryocytosis inhibition (EPL). Vet. Microbial. 1978 (in
press)
10. Kettmann. R., Burny. A.. Cleuter, Y., Ghysdael. J., Mammerickx.
M.: Distribution of bovine leukemia virus proviral DNA sequences
in tissues of animals with enzootic bovine leucosis. Leukemia Research
2/23-32 ( 1978) II. Mammerickx. M., Burny, A.. Dekegel. D.. Ghysdael.
J.. Kettmann. R.. Portetelle, D.: Comparative study of four diagnostic
methods of enzootic bovine leukemia. Ln: Bovine leucosis: Various
methods of molecular virology. Burny. A. (ed.). pp. 209-221. Luxembourg:
Commission of the European Communities 1977
12. Mammerickx. M.. Otte. J.. Rase. F.. Braibant. E., Portetelle.
D.. Burny. A.. Dekegel. D.: Large scale serological detection in
Belgium of enzootic bovine leukosis. Zbl. Vet. Med. B 25, 416-424(1978)
13. McDonald. H. C.. Ferrer, J. F.: Detection. quantitation and
characterization of the major internal virion antigen of the bovine
leukemia virus by radioimmunoassay. .r. Natl. Cancer Inst. 57,875-882
(1976)
14. Olson. C.. Baumgartener. L. E.: Pathology of Lymphosarcoma in
sheep induced with bovine leukemia virus. Cancer Res. 36, 2365-2373
( 1976 )
15. Paul. P.S.. Pomeroy. K.A.. Castro. A.E., Johnson. D.W.. Muscoplat.
C.C.. Sorensen. D.K.: Detection ofbovine leukemia virus in B-Iymphocytes
by the syncytia induction assay. .J. Natl. Cancer Inst. 59, 1269-1272
(1977)
16. Portetelle. D.. Bruck. C.. Bex. F.. Burny. A.. Dekegel. D..
Mammerickx. M.: Detection or complement dependent lytic antibodies
in sera from bovine leukemia virus infected animals by the chromium-51
release assay. Arch. internat. Physiol. Biochim. 86,955--956 (1978)
17. Takashima, I.. Olson. C.. Driscoll. D. M., Baumgartener. L.
E.: B lymphocytes and T lymphocytes in three types of bovine lymphosarcoma.
J. Natl. Cancer Inst. 59, 1205-1210 ( 1977)
18. Van der Maaten. M..J.. Miller. J. M.. Boothe. A. D.: Replicating
type-C virus particles in monolayer cell cultures of~ tissues from
cattle with lymphosarcoma. J. Natl. Cancer Inst. 52, 491-497(1974)
19. Wittmann, W.. Urbaneck. D.: Leukosen dies Rindes. Ln: Handbuch
der Virus-Infektionen bei Tieren. Fischer. I. (ed.). Vol.V. pp.
41-174. 1969 20. Zavada. J.. Dickson. C.. Weiss. R.: Pseudotypes
of vesicular stomatitis virus with envelope antigens provided by
murine mammary tumor virus. Virology 82,221-231 ( 1977)
|





