|
Institute of Cancer Research, Chester Beatty Laboratories, Fulham Road, London SW3 6JB, England Like other enveloped viruses, the outer membrane antigens of retroviruses present target antigens for neutralizing antibodies, and also playa key role in interacting with cell-surface receptors for initial stages of infection. We have investigated these attributes of the envelopes of human T -lymphotropic retroviruses (Weiss 1985; Wong-Staal and Gallo 1985) and our studies are briefly summarized here. The viruses investigated are human T -cell leukemia virus type-1 (HTL V -1 ), the etiologic agent of adult T -cell leukemia-lymphoma (A TL), HTL V -2, first isolated from the leukemic cells of a patient with T -cell hairy leukemia (Kalyanaraman et al. 1982) and present in a proportion of intravenous drug abusers (Tedder et al. 1984), and human immunodeficiency virus (HIV -1, HTL V-3/LA V) the causative agent of AIDS.
HTL V -1 and HTL V -2 are not readily amenable to quantitative titration of infectivity because the virions are largely cell-associated. We have therefore employed a viral pseudotype technique for detecting neutralizing antibodies and for probing receptors. The pseudotypes are virions of vesicular stomatitis virus enveloped by the glycoproteins of HTLV-1 or HTLV-2 (Clapham et al. 1984), a system originally devised for avian retroviruses by Zavada (1972). Pseudotypes possess the neutralization and host range properties of the retrovirus, while replicating as VSV to produce cytopathic plaques in cell monolayers that can be assayed in a simple, quantitative way. Using stocks of VSV(HTLV-1), we found that sera of infected subjects, whether healthy or suffering A TL, possessed neutralizing titers ranging from 1 : 50 to 1: 50000 (Clapham et al. 1984). In collaboration with H. Hoshino, we showed that VSV(HTL V-1) pseudotypes prepared with J apanese or American HTL V -1 isolates did not distinguish by neutralization titer between sera from Japanese and British West Jamaican subjects (Hoshino et al. 1985). For HTL V -2 neutralization, we have used a single isolate from an American patient (Kalyanaraman et al. 1982) for pseudotype preparation, and observed high neutralization titers in the sera of 4% of British intravenous drug abusers (Tedder et al. 1984). There is a slight degree of cross-neutralization between HTL V -1 and HTL V -2, but the heterologous titers are 100- to 1000-fold lower than the homologous titers (Clapham et al. 1984).
Table 1. Common and strain-specific neutralizing
activities of human and rabbit sera to HIV -1 a 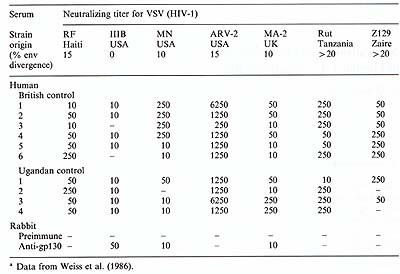
C. Common and Variable Antigens for HIV -1 Neutralization We have also detected neutralizing activity in sera of subjects infected with HIV -1 (Weiss et al. 1985 a, 1986). In a longitudinalstudy of initially asymptomatic male homosexuals known to be infected with HIV-1 for at least 3 years (Weber et al. 1987), the continued presence and a slightly rising titer of neutralizing anti-HIV-1 appears to correlate with a relatively good prognosis. However, a stronger correlation was found with low or no anti-gag serum activity (measured by radioimmunoprecipitation) and progression to AIDS or AIDS-related complex (ARC). We do not, at present, know whether some of the neutralizing activity may be directed to gag antigens, though this appears unlikely for VSV(HIV-1) pseudotypes, which are thought to assemble only membrane glycoproteins of the retrovirus. Using VSV(HIV-1) pseudotypes prepared with seven different isolates of HIV -1 from USA, Haiti, Europe and Africa, we examined the specificity and variability of neutralizing epitopes (Weiss et al. 1986). Sera from Ugandan and British subjects selected for capacity to neutralize the RF (Haitian) isolate, showed significant neutralizing activity for almost all HIV -1 strains (Table 1 ). Interestingly, the AR V -2 pseudotype stock appeared to be most sensitive to neutralization, whatever the origin of the anti-HIV human serum. By contrast to the cross-neutralization observed with human sera, we found in collaboration with L. Lasky and P. Berman that a rabbit serum raised by Lasky et al. (1986) against a recombinant gp130 molecule specific to the outer envelope glycoprotein of HIV -1, neutralized the strain from which the recombinant was made (HTL V -IIIB), but only two of the other six strains. Even the AR V -2 isolate that was so sensitive to human sera was not significantly neutralized by the anti-gp130 rabbit serum. Tests with further rabbit and guinea-pig sera raised against gp130 confirmed the strainspecificity of this antigen (Weiss et al. 1986). The antigen targets for neutralization of HIV -1 thus appear to include epitopes common to widely divergent HIV -1 strains as well as variable antigens specific to individual strains and more closely related isolates. The presence of common neutralization antigens between HIV -1 strains differing by 20% or more in the envelope gene (Alizon et al. 1986; Starcich et al. 1986) is a promising finding for the development of vaccines.
HIL V sand HIV share a common property of tropism for I4+ lymphocytes in vivo (Weiss 1985; Wong-Staal and Gallo 1985). AIL is a I4+ neoplasm, while the salient feature of AIDS is the depletion of T4 + T helper cells. However, other cell types can be infected in vitro by HTL V -1 (Clapham et al. 1983) and by HIV-1 (Levy et al. 1985; Gartner et al. 1986). We investigated to what extent the cellular tropisms of HTL V and HIV are determined by the cell surface receptors for initiating infection, and identitied the T4 (CD4) antigen itself as an important component of the HIV -1 receptor (Dalgleish et al. 1984). Using VSV pseudo types with envelopes of HIL V or HIV, we found that HTL V -1 and HIL V -2 recognise receptors on diverse types of human cells and mammalian cells of many species. Productive infection of cells by HTL V -1 not only blocks receptor availability to VSV(HTLV-1) but also to VSV(HTL V -2), indicating that these two viruses use a common receptor (Weiss et al. 1985b). The recognition ofa common cell surface receptor for HTLV-1 and HTLV-2 accords with the slight cross-neutralization of these viruses already noted, as there may be a common epitope of the external glycoprotein that binds to the receptor. We do not at present know the biochemical nature of the HTL V receptor . Pseudotypes of HIV -1 plate only on cells expressing T4 antigen (Dalgleish et al. 1984). These may be T -helper lymphocytes or monocytes, such as the U937 cell line. Using monoclonal antibodies (mAbs) to T4 antigen, we found that pseudotype infection, and also induction of multinucleated syncytia by HIV -1, could be blocked in T 4 + T cells (Dalgleish et al. 1984) and U937 cells (Clapham et al. 1987). Infection by HIV-1 (Dalgleish et al. 1984) and treatment by TP A (Clapham et al. 1987) caused concomitant disappearance of T4 antigen and HIV-1 receptor from the cell surface without affecting the HTL V receptor. Other groups have also observed that mAbs specifically blocked infection by HIV -1 (Klatzmann et al. 1984) and binding of labelled HIV-1 virions (McDougal et al. 1986). Preliminary studies also indicate that HIV -2 (LA V -2; Clavel et al. 1986) reception is also blocked by certain anti- T4 mAbs. Thus, the T4 antigen acts as a specific receptor on lymphocytes and monocytes for binding HIV. The precise epitopes on the T4 antigen recognized by HIV -1 remain to be determined. In collaboration with Q. Sattentau and P. C. L. Beverley, we have analyzed a series of25 different mAbs raised against T4 for ability to block each other and HIV -1 syncytium induction (Sattentau et al. 1986). The mAbs that interfere with HIV -1 reception fall into three noncompeting groups. Two block HIV -1 completely and the third only weakly. Some mAbs, e.g., OKT4 and OKT4c, fail to compete with the HIV-1 receptor at all. These epitopes are thought to be located near to the transmembrane domain of the T4 molecule. Although there is some degree of variation in T4 epitope expression on T lymphocytes from different African and Caucasian individuals, this was not correlated with susceptibility to HIV -1 infection in vitro and did not affect those epitopes most important for T4 binding (Sattentau et al. 1986). We next examined whether the T4 antigen would act as a functional receptor for HIV -1 if expressed ectopically on the surface of unusual cell types. In collaboration with P . Maddon, R. Axel, and J. S. McDougal, the cDNA for the human T4 gene was introduced into various human and mouse cell types via a retrovirus vector carrying a neomycin resistance gene as a dominant selectable marker (Maddon et al. 1986). Our findings, summarized in Table 2, show that human cells such as the immature T -cell line HSB2, the Burkitt's lymphoma line, Raji, and the cervical carcinoma line, HeLa, become susceptible to HIV -1 infection and replication upon expression of T4. On the other hand, transfection and expression of the human T4 gene to mouse cells, including L3T4+ mouse cells (the murine equivalent of human T4 + lymphocytes), does not result in infection of HIV-1 or of the VSV(HIV-1) pseudotype. HIV -1 virions did, however , bind specifically to the surface of T4 transfected mouse cells and not to control mouse cells. Therefore, we conclude that T4 antigen is sufficient for HIV -1 attachment to the surface of mouse cells, but that some other human component, present in HeLa and Raji cells, is necessary for functional penetration following attachment. It appears that the binding of virions alone is insufficient to trigger endocytosis of the ligand-receptor complex on mouse cells. Table 2. Interaction of HIV -1
with cells transfected with the human T 4 gene a 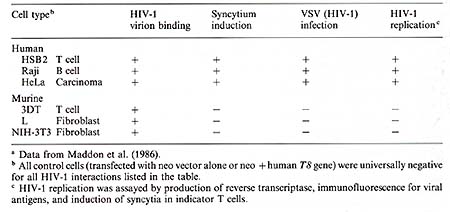
E. Conclusions Our studies of virus neutralization serve to distinguish different strains of human retroviral pathogens and may eventually provide prognostic tools and aid the development of vaccines. Our receptor studies serve to distinguish the cellular tropisms of HTL V sand HIV s, and have identified certain epitopes of the T4 surface antigen as the binding receptor for HIV. Infection of T4+ lymphocytes and monocytes by HIV -1 largely explains the immunodeficiency underlying the opportunistic infections and neoplasms evident in AIDS. The binding of free envelope glycoprotein to uninfected T4+ cells when shed by infected cells may further exacerbate the immunodeficiency, as T4 antigen plays a functional role in cell interactions in the immune system (Dalgleish 1986).
|