|
* Laboratory of Biochemical Pharmacology, Dana-Farber
Cancer Institute, Department of Pathology,
Harvard Medical School, Department of Cancer Biology Harvard School
of Public Health
A. Introduction
Human retroviruses represent an emerging class of complex pathogens
involved in a wide variety of maladies, including leukemias and
lymphomas, diseases of the central nervous system, and immune function
impairment. These have recently been reviewed by Wong-Staal and
Gallo. Four different types of human retroviruses have been isolated
to date: the etiological agents of a malignant T cell leukemia/lymphoma,
the virus HTL V -I which causes the disease A TLL, two viruses associated
with more benign forms of T-cellleukemia (HTLV-II), and the etiological
agent of the acquired immune deficiency syndrome and related disorders
(HIV). Additionally, retroviruses of genomic organization similar
to that of HIV but differing markedly in DNA sequence have recently
been isolated among persons in West Africa (Kanki et al. 1985; Clavel
et al. 1986). As far as they have been characterized to date, the
human retroviruses display interesting features of growth regulation
not previously observed for the well characterized murine and avian
retroviruses. The following presents a brief overview of the some
of the unusual features of human leukemia viruses with some discussion
of similar features in the bovine leukemia virus and simian T -cell
leukemia virus, which have genomic organization similar to that
of the human T -cell leukemia viruses.
B. Pathogenesis
The T -cellleukemia and lymphoma induced by HTL V -I and -II all
appear only after a very long incubation period, measured in decades
(Catovsky et al. 1982). Infection is marked by seroconversion, but
there is some evidence that seroconversion may occur only after
very prolonged periods, ranging from 10 to 15 years from the time
of infection at birth to the time of seroconversion in the teens.
There is an absence of viremia in the patients and a notable lack
of virus expression even in fresh tumor cell populations (Franchini
et al. 1984). Stimulation of infected patient T cells with mitogens
results in the expression of high levels of viral RNA and protein
and the budding of virus particles (Poiesz et al. 1980).
T cells from infected patients can be made to transform normal peripheral
blood T cells from uninfected people (Chen et al. 1983; Popovic
et al. 1983; Miyoshi et al. 1981; Yamamoto et al. 1982). Such transformation
is generally accomplished by co-cultivation and is very difficult
to accomplish with cellfree virus. The transformed cells have the
appearance of tumor cells, characterized both by a distinctive set
of surface markers including the T4 antigen and by large lobulated
nuclei similar to those of the tumor cells. The fresh tumors cells
and celllines immortalized by HTL V -I express abnormally high levels
of the interleukin 2 (IL-2) surface receptor.
The absence of viremia in infected persons and the difficulty of
free infection may help to explain the epiderniology of infection
transmission. For most populations, including those in the Pacific
rim, particularly Japan and Taiwan, and in the Caribbean, Africa,
and the United States, transmission is limited to family contexts
(Blattner et al. 1983). Transmission frorn mother to child and frorn
infected male to fernale partner is documented, whereas transmission
frorn infected fernale to male sex partners is thought to be rare.
The virus is also transmitted by needle, either by blood transfusion
or by hypodermic syringe. The latter route appears to be a significant
factor in current transmission patterns of the virus, as large proportions
of certain populations for instance, intravenous drug abusers havebeen
found to be infected with either HTLV-I, HTLV-II, or HTLV-IV, depending
upon the geographical region.
C. Genomic Organization
How might one explain the limited replication and the pathogenesis
of these viruses in molecular terms?
The genomic structure of the human leukernia viruses differs frorn
that of other retroviruses characterized to date except for the
two very close relatives of these viruses, the simian T -cell leukernia
virus type I (STLV-I) and, more distantly, the bovine leukernia
virus (BL V). The latter, like HTLV-I, -II, and -V, is poorly infectious,
and it is transmitted most commonly by the veterinarian needle.
The unusual features of the organization of these viruses is pictured
in Fig. 1. As with all other retroviruses the human leukemia retroviruses
contain genes that encode the virion internal capsid proteins (gag
gene proteins), genes that encode replication functions (reverse
transcriptase, integrase, and protease), and genes that specify
the exterior proteins which are embedded in the lipid layer that
surrounds virion. The envelope protein is comprised of an exterior
glycoprotein ,nd an integral transmembrane protein. The organization
of the virion structural genes and replicative genes is similar
to that of the simplest avian, murine, and feline viruses. 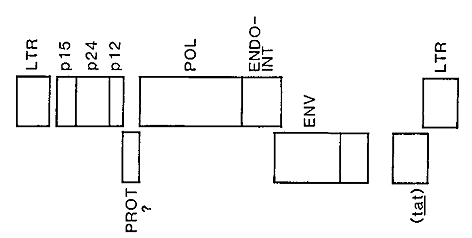
Fig. 1. Provirus structure of HT L V -I
The genome of HTLV-I, HTLV-II, and BLV viruses differs frorn that
of other retroviruses by the presence of approximately 1500 nucleotides
located between the 3' end of the envelope glycoprotein and the
3' LTR (Iong terminal repeat) (Seiki et al. 1983; Haseltine et al.
1984; Shimotohno et al. 1984). This region, called pX, has the capacity
to encode multiple polypeptides of the size of 100 amino acids or
greater. For an analysis of the coding capacity of the pX region
of HTL V -I, see the review by Haseltine et al. (1984). Similar
analyses indicate that the corresponding regions of HTL V -II and
of BL V have the capacity to encode numerous polypeptides.
It has been demonstrated that the pX region of HTL V -I specifies
at least three polypeptides which are made in infected, activated
T cells (Kiyokawa et al. 1985). The largest of these proteins of
sizes 42 kD,38 kD, and 36 kD for HTLV-I, II, and BLV, respectively
encode a protein that is 10cated primarily in the nucleus (Goh et
al. 1985; Slamon et al. 1985). Initially we have called this protein
the X -lor protein for the product of the long open reading frame
within the X region; however, we now refer to it as the tat gene
product for tran.s-activator (see below) (Sodroski et al. 1985b).
A subscript, tatI tatII or tatBLV or tatsTLV, denotes the virus
of origin. Approximately half the people infected with HTLV -I,
whether symptomatic or not, produce antibodies to this protein.
The tat protein is also called X or pX40 by others who have confirmed
the existence of this gene product in HTLV- I and -II infected cells
(Felber et al. 1986; Seiki et al. 1986).
The tat product is synthesized frorn a doubly spliced messenger
RNA specieswhich includes transcripts of portions of the 5' L TR,
a small sequence located immediately 5' to the envelope gene, and
the distal two-thirds ofthe pX region through the end of the 3'
L TR (Sodroski et al. 1985 b; Seiki et al. 1985; Wachsman et al.
1985; Aldovini et al. 1986).
It has recently been reported for HTLV-I that this same messenger
RNA species encodes two other polypeptides frorn an overlapping
reading frame (Fig. 1) (Kiyokawa et al. 1985; N agashima et al.
1986). The initiating codons for the larger of these two polypeptides
is located 5' to the site of initiation of the tat gene product.
The same splice donor-acceptor combinations as is used for production
of the tat gene product places the alternative open reading frame
in the correct register with a second open reading frame which overlaps
that used to produce the tat gene product. The product ofthis second
initiation event is a 27 kD protein. The protein is phosphorylated
and located predominantly in the nucleus (Kiyokawa et al. 1985).
The protein is called pp27, denoting both its size and the observation
that it is phosphorylated. A second polypeptide is also synthesized
frorn the same reading frame as is the pp27 protein. This third
product ofthe pX region is thought to be initiated at an AUG codon
within the second coding exon of the messenger RNA. This protein
is also phosphorylated and has an apparent molecular weight of 21
kD; it is located primarily in the cytoplasm.
lt is notable that the genomes of HTLV-II (Shimotohno et al. 1985),
STLV-1 (Watanabe et al. 1985), and BLV (Sagata et al. 1985 a, b)
all possess the capacity to encode similar alternative reading frame
polypeptides. lndeed, there is evidence that BLV, as does HTLV -1,
in fact also encodes such proteins (Yoshinaka and Oroszlan 1985).
I t is acurosity that these proteins do not raise antibodies in
infected people. No reactivity to these smaller proteins is observed
in cattle or sheep infected with BL V. The complete coding capacity
of these virus has not yet been fully explored. lt is conceivable
that other virally encoded proteins which are of low antigenicity
in infected people are present in virus-infected cells.
D. Trans-Activation: The tat Protein
The phenomenon of trans-activating retroviral gene products was
first reported for HTLV-I and -11 (Sodroski et al. 1985b). lt was
observed that the LTRs of HTLV-I and -II function much more efficiently
as promoter elements in infected than in uninfected cells (Fig.
2) (Sodroski et al. 1985 b ). A positive tran.s-activating genetic
regulatory system requires at least two elements, the tran.s-activator
product and a ci.s-acting responsive element.
The tran.s-activator product of HTL V -II was initially identified
as the product of the HTLV-II pX open reading frame (Sodroski et
al. 1985 a). lt has since been reported that the pX open reading
frame of HTL V-I and of BL V also encodes a trans-activator (Rosen
et al. 1986). lsogenic celllines which differ only in their ability
to express the long open reading frame product are capable of trans-activation.
Gene expression directed by a plasmid which carries the tran.s-activator
gene has been shown to stimulate the homologous LTR of HTLV-I, -11,
and BLV (Sodroski et al. 1985 a; Pashkalis et al. 1986; Rosen et
al. 1986; Fujisawa et al. 1986). Plasmids constructed so as to eliminate
the possibility of producing the pp27 and pp21 gene products are
also capable of trans-activation as measured in transient cotransfection
assays (Kiyokawa et al. 1985). The L TR of HTL V -I can also be
activated by the tat gene product ofeither HTL V-I or -II but not
by the tat BL V product (Sodroski et al. 1985 a; Rosen et al. 1986).
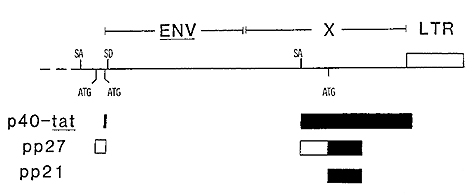
I -Fig.2. HTLV-I X region illustrating the openreading
frames known to encode protein
The increase in LTR-directed gene expression induced by the tat
genes is accompanied by an increase in the steady-state level of
corresponding messenger RNA species (Sodroski et al. 1985 b; Felber
et al. 1985). The increase in messenger RNA levels of heterologous
genes directed by the L TR corresponds very roughly to the level
of increase observed for protein expression. However, precise correspondence
is difficult to document and post-transcriptional alterations in
the efficienty of mRNA utilization cannot be ruled out entirely.
A cautionary note is in order. At present trans-activation ofviral
genes is only inferred from the ability of the trans-activator proteins
to increase the expression of heterologous genes directed by the
HTL V L TRs. Direct induction of viral genes by the trans-activators
has not yet been reported. Therefore the ability of the tat gene
alone to stimulate the expression of viral genes is not established.
The cis-acting regulatory sequences, called TAR (trans-acting responsive
region), were initially found to be located in the U3 region of
the viral TR, entirely 5' to the site of initiation of viral RNA
synthesis (Rosen et al. 1985). It was noted above that the U3 element
of the HTLV-I and -II LTRs contained 21 nucleotide sequences repeated
several times and that the sequences ofthese repeat units were preserved
between HTLV-I and -II (Sodroski et al. 1984). It was also observed
that except for these repeated sequences and for a short region
near the site of RNA initiation, the sequences of the HTLV-I and
-II LTRs are notably different as compared to the extent of conservation
of other parts of the genomic sequences. Recently synthetic oligonucleotides
which correspond to these 21-nucleotide long sequences have been
demonstrated to convey a response to the tran.s-activator upon heterologous
promoters (Shimotohno et al. 1986). The response to the trans-activator
is observed when the 21-nucleotide repeat sequences are located
proximal to the promoter and is irrespective of the orientation
of the 21-nucleotide sequence with respect to the promoter. In some
experiments a single repeat unit suffices to convey the trans-activation
response (Rosen et al., to be published) whereas others report that
two or more 21-nucleotide long sequences, tandemly repeated, are
required for the transactivation effect. These repeat units are
called T AR-21 sequences to denote the observation that they convey
a responsive phenotype (Rosen et al., to be published).
Another cautionary note is appropriate, Although the TAR-21 sequences
do permit increased expression of both homologous and heterologous
promoters in the presence of the trans-activators, the response
is weak and the level of expression of heterologous genes is one
or two orders of magnitude below that observed for promoters and
their natural configuration even for promoters which contain 5'
deletions that preserve only the TAR-21 sequence located proximal
to the promoter (Rosen et al. 1985). This observation suggests that
promoter strength and inducibility depend upon the sequences adjacent
to TAR-21.
Two other curious features of the viral promoters are notable. The
promoter strength of HTLV-I is dependent upon sequences located
3' to the site of RNA initiation, within the Rand U5 regions of
the LTR (Derse and Casey 1986; Rosen et al. , to be published).
A set of nested deletions originating in the U 5 region of the HTLV-I
L TR and extending to the site of RNA initiation results in a progressive
weakening of promoter activity. Gene expression directed by such
altered L TRs is inducible by the transactivator genes, although
the ultimate level of L TR -directed gene expression is progressively
diminished both in the induced and uninduced states by these deletions.
Evidently the Rand U3 region of the viral L TR encodes sequences
important for high-level L TR-directed gene expression. Location
of these sequences 3' to the site of RNA initiation raises the possibility
that they may be involved in post-transcriptional regulatory events
as weIl as in contributing to the rate of RNA initiation. Sequences
which have similar effects are reported to exist 3' to the site
of RNA initiation within the BL V LTR.
The second notable feature of the viral LTRs lies in asymmetry in
the function of the HTLV-I and -ll sequences. The LTR of HTLV-I
functions well as a promoter of heterologous genes in a wide variety
of cell types, unrestricted as regards species or tissue of origin
(Rosen et al. 1985). The activity of the HTL V -II L TR is markedly
limited (Sodroski et al. 1985). lt functions well in very few cell
types. lt is remarkable that the HTLV -ll LTR does not function
as a promoter in most human lymphoid cell lines, whether they be
Tor B cells. In fact, no promoter activity was observed in two human
lymphoid cell lines that expressed a functional tatII product (Sodroski
et al. 1985 a). The tatll product in these celllines was found to
be capable of stimulating the HTLV-I L TR, while in the same celllines
no HTLVII promoter activity was observed. lt can be concluded that
the HTLV -ll promoter is either extremely fastidious as regards
the requirement for cell-specified expression factors or that viral
gene products of the transactivator are required for activity of
the HTLV-II LTR. Such other gene products cannot be supplied by
the alternative reading frame product, pp27, as the HTLV-II LTR
is inactive in the cell line that is reported to express both the
HTLV -1 trans-activator and pp27 proteins. The BLV LTR also displays
a narrow cellline activity and is a very poor promoter in most uninfected
cell types.
E. Transactivation:
The pp27 and pp21 Proteins
A recent report by lnoue et al. (1986) indicates that the pp27
protein may play an important role in virus replication via a tran.sacting
mechanism. An integrated provirus deleted for the amino terminal
portion ofthe env gene was found to be defective for RNA synthesis
and for gag gene production. The deletion was such as to eliminate
the 5' coding exons of the tat and pp27 proteins. Transfection of
a cellline containing this defective provirus with plasmids capable
of expression of the tat and/or pp27 proteins revealed that gag
gene synthesis was dependent upon both tat and pp27 gene expression
from the transfected plasmids. Moreover, no gag gene RNA was detected
upon transfection with the tat expressing plasmid alone.
This observation indicates that both the tat and pp27 proteins are
needed for the expression of viral genes. Heterologous gene synthesis
directed by the HTLV-I LTR, however, is not dependent on pp27, nor
does the expression of pp27 markedly affect the rate of expression
of such constructs (Rosen, Sodroski, Dokhelar, and Haseltine, unpublished
observations).
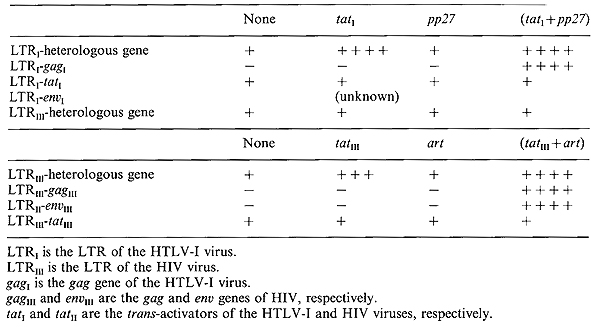
The function of the pp27 gene resembles in a formal sense that of
the art gene of HIV (Table 1). Neither pp27 nor art are required
for expression of heterologous genes under the control ofthe LTR.
However, in the absence of a second gene product the trans-activator
genes (tat genes) of these viruses are insufficient to permit expression
of viral gag proteins. We nevertheless note that the trans-activator
genes of both viruses can be synthesized in the absence of auxiliary
proteins (Rosen, Sodroski, Dokhelar, and Haseltine, unpublished
observations). For both viruses the regulatory genes are controlled
independently frorn the structural genes.
Although the art and pp27 genes display a formal analogy in functional
terms, such similarity does not necessarily imply that the mechanism
of action is the same. The transactivator gene of HTL V-I acts primarily
as a transcriptional tran.s-activator of the viral LTR whereas the
tran.s-activator of HIV is primarily a post-transcriptional activator.
It rernains to be tested whether the pp27 protein possesses an antirepression
function as does art, although the preliminary genetics suggest
that this is likely. Table 1 also shows that the tat genes of HTLV
-I and of HIV do not reciprocally tran.s-activate the heterologous
virus LTRs.
Table 1
F. The Mechanism of Transformation
The process of in vitro formation of tumors by HTL V-I, -II, and
BL V has not been fully characterized. Infection of T cells by the
virus does not result in immediate tumor formation. Rather, tumors
arise rarely (1 in 100- 300 infected people over a lifetime ). The
role ofviral genes in the transformation process is strongly inferred
by epiderniological studies which link seropositivity to disease
as weIl as the observation that T-cell tumors in infected people
invariably contain at least one integrated copy of the provirus
(Seiki et al. 1984). It is sometimes observed that tumors contain
only the 3' portion of the genorne. However, most of the tumors
found in patients contain, as a minimum, the 5' L TR and the pX
region.
T -cell tumors in patients are clonal with respect to the site of
integration of the provirus (Seiki et al. 1983; Hahn et al. 1983).
The long latent period and the clonal natureof the tumors indicate
that events in addition to infection of T cells with the virus are
required for the appearance ofmalignant tumors. Such events may
represent either secondary changes occurring within the infected
cell, such as somatic mutations, or changes in the immunological
status of the host.
Two additionalobservations indicate that the viral genes play an
important role in the initiation and maintenance of tumors. Tumorigenesis
by the avian, murine, and feline retroviruses which contain only
those genes required for virion formation and virus replication
depend upon activation of cellular growth regulatory genes. This
conclusion is reached frorn the observation that independent, virally
induced tumors contain proviruses that are found integrated near
the same cellular genes. Such is not the case for tumors induced
by HTLV-1 or BLV, for which no repeated chromosomal sites of integration
have been observed in naturally occurring tumors (Seiki et al. 1983;
Hahn et al. 1983). I t is theref ore inferred that viral genes thernselves
playa key role in the initiation and maintenance of the tumor phenotype.
The role of the viral genes in the transformation process is also
inferred frorn in vitro transformation studies. Primary T cells
can be immortalized by co-cultivation with
infected cells treated with mitomycin C. In contrast to role cultures,
recipient cell cultures continue to proliferate without continued
antigen stimulation in the presence of the T -cell growth factor,
IL-2. Eventually immortalized cells ernerge frorn such cultures
(Chen et al. 1983; Popovic et al. 1983; Miyoshi et al. 1981; Yamamoto
et al. 1982). The expanding population of T cells is initially polyclonal
with respect to the site of provirus integration. Cell lines that
are monoclonal with respect to the sites of viral integration eventually
ernerge frorn the population and dominate the culture. Such cell
lines may rernain dependent upon IL-2 for growth or may become capable
of IL-2 independent growth, depending on cell culture conditions.
Such immortalized primary cells are typically T4+ cells as are most
HTLV-I induced tumors. T8 + cell lines can be derived by co-cultivation
ofmitomycin-treated infected ce]ls with primary populations of Iymphocytes
enriched for cells which bear the T8 antigen (DeRossi et al. 1985).It
is possible that cell lines established from patient cells are not
derived from tumors themselves but represent immortalization of
the normal patient T cells by a mechanism analogous to that described
for immortalization ofT cells via co-cultivation. In this regard
Waldmann and colleagues have found the T -cell receptor beta gene
rearrangement in patient and tumor cells to differ (T. Waldmann,
personal communication).
Events that occur between the initiation of infection and establishment
of IL-2 dependent or independent T -cell lines have not been weIl
characterized. Selection of specific fast growing clones may occur
both in infected patients as weIl as in vitro. It is possible that
secondary changes occur within the infected cell which permit rapid
growth. AIternatively, the clonality of the tumor cells may represent
selection of a cell population which expresses high levels of viral
proteins that promote cellular growth.
G. lnduction of the IL-2 Receptor by the Trans-activator Gene
The promoter of the IL-2 receptor and the IL-2 genes have been
cloned. Cotransfection ofthe promoters placed 5' to reported genes,
such as the chloramphenicol acetylase transferase gene, with the
trans.-activator gene of HTL V -I has been shown to increase the
level of expression of the IL-2 gene promoter (W. Greene, personal
communication and our unpublished observations). The level of expression
ofthe genes under the control ofthe IL-2 promoter was found be increased
slightly in similar experiments. The trans-activator gene of HTLV-II
has also been shown to increase the level of expression of the IL-2
receptor gene, albeit more weakly than that observed for the tatI
gene, at least in the particular experimental configuration used.
These observations suggest that the transactivator gene ofHTL V-II
can contribute to the growth properties of the T cell by deregulation
of genes which normally control T -cell proliferation in response
to antigen stimulation. Such a model for T -cell transformation
must include the additional consideration that the expression of
the viral genes is dependent upon T -cell activation. Thus, an infected
resting T cell should not be transformed as the viral genes are
not expressed.
Although simple, this explanation for transformation does not suffice
to account for the clinical observations with A TLL patients. If
tat genes were sufficient to induce both IL-2 and IL-2 receptors,
infection should lead to transformation. However , malignant growth
of T cells in infected patients is a rare event. It is also possible
that the pp27 and pp21 proteins playa role in the activation of
cellular genes.
H. Summary
The broad outlines of mechanisms of tumorigenesis by the HTL V
-I family of viruses are beginning to emerge. The viruses encode
at least three genes in addition to the genes (gag, pol, and env)
required for virus replication. These additional genes encoded for
by the X region are likely to affect in a specific fashion the growth
of lymphocytes. The tat gene appears to mimick at least part of
the response of mature lymphocytes to recognition of the cognate
antigen. That is, in T lymphocytes the tatI gene seems to induce
the IL-2 and IL-2 receptor genes (W. Greene et al. 1986). The alternative
reading-frame proteins, pp21 and pp27, have some similarity of cellular
proteins that are associated with Go to Gl transitions and may contribute
to the transformed phenotype in cooperation with the tat gene. The
expression of viral genes in infected lymphocytes, the tat gene
and pp21 and pp27 proteins, and possibly other viral genes (since
the coding capacity of the X region is not exhausted by the tat
and pp21 and pp27 proteins) may be sufficient to account for the
transformation of T cells in culture. A secondary change in the
infected cells in culture is not required to explain the outgrowth
of cells which are clonal with respect to the site of viral genomic
integration, as selection of the most rapidly growing infected cell
could account for this observation. The case of infected patients
is more complex. Infection of T cells with the HTL V -lor -II virus
is not sufficient to produce malignant disease. Failure of the virus
to induce malignancy in all infected T cells may be attributed to
diverse causes. It is possible that viral gene expression is suppressed
in most infected T cells. Certainly no viral RNA is detected in
peripheral lymphocytes of infected patients which include the tumor
cells themselves. Transcriptional repression of viral genes in infected
cells is a sufficient explanation for the failure of the virus to
transform most T cells in patients. It is also possible that T cells
which do express viral antigens are eliminated by the immune system.
The observation that many tumor cell lines derived from patients
contain deletions of virus structural proteins is consistent with
this notion. Patients infected with HTL V -I and -II do show good
immune responses to virion structural proteins. An additional explanation
may lie in homeostatic regulatory mechanisms of the immune response
itself. Lymphocytes are thought to possess regulatory mechanisms
that limit their proliferation response to antigen recognition.
The early proliferative response of T cells in response to the presence
of the cognate antigen is followed by reestablishment ofa resting
phase. Stabilization of the stimulated population of T cells was
thought to involve activation of an internal cellular program of
a repressive nature. Interaction of the activated T cells with other
components of the immune system may also contribute to reestablishment
of the resting state. It is conceivable that the homeostatic mechanisms
regulating T -cell proliferation also regulate HTL V -I and -II
gene expression and thereby limit the growth of infected cells in
patients. In this view, malignant transformation by HTLV-I and -II
requires bypass of the normal homeostatic mechanisms of growth control
of lymphocytes. Such bypass may occur either by a secondary intracellular
change that occurs in the infected cells or it may be due to a systemic
failure of normal immunoregulatory mechanisms. Either process could
give rise to a tumor cell population, the first by outgrowth of
a cell which contains a secondary genetic lesion, and the second
by overgrowth of the infected cell population by fast growing infected
cells as is observed in cell culture. The molecular biology and
in vivo replication of the virus also provide some insight into
the mechanisms of transmission into the virus. This family of viruses
seems to be either poorly infectious or altogether noninfectious
for uninfected cells. For establishment of infection it is likely
that viral gene products transferred from an infected cell by cell
fusion are required. The infectious unit may well be an infected
cell rather than a cell previrion. In this context the X genes of
this family of viruses are required for replication and may be viewed
as replicative genes. Tumorigenesis may be a byproduct of the natural
replicative cycle of this family of viruses.
References
1. Aldovini A, De Rossi A, Feinberg MB. Wong-Staal F, Franchini
G (1986) Molecular analysis of adeletion mutant provirus of type
I human T -celllymphotropic virus: evidence for a doubly spliced
x-lor mRNA. Proc Natl Acad Sci USA 83:38-42
2. Blattner W A et al. (1983) J Infecl Dis 147:406-412
3. Clavel F et al. (1986) Science 233:343-346
4. Calovsky D et al. (1982) Lancet 1:639-643
5. Chen IS et al. (1983) Proc Nall Acad Sci USA 80:7006-7009
6. De Rossi A et al. (1985) Virology 163.640-645
7. Derse D, Casey JW (1986) Science 231.1437-4411
8. Felber BK, Paskalis H, Kleinman-Ewing C, Wong-Staal F, Pavlakis
GN (1985) Science 229.675-679
9. Franchini G et al. (1984) Proc Natl Acad Sci USA 81 :6207-6211
10. Greene W et al. (1986) Science 232:877
11. Hahn B et al. (1983) Nature 305:340-341
12. Haralabos P, Felber BK, Pavlakis GN (1986) Cis-acling sequences
responsible for the transcriptional activation of human T -cell
leukernia virus type I constitute a conditional enhancer. Proc Natl
Acad Sci USA 83:6558-6562
13. Haseltine W A et al. (1984) Science 225:419-421
14. Inoue JI, Seiki M, Yoshida N (1986) Febs Lett 209:187-190
15. Josephs SF, Wong-Staal F, Manzari V, Gallo RC, Sodroski JG,
Trus MD, Perkins D, Patarca R, Haselline W A (1984) Long terminal
repeat structure of an American isolaIe of type I human T -cell
leukernis virus. Virology 139:340-345
16. Kanki PJ et al. (1986) Science 232:238-243
17. Kiyokama T, Seiki M, Iwashita S, Imagawa K, Shimiza F, Yoshida
M (1985) p27x-III and p21 x-III, proteins encoded by the pX seq
uence of human T -cellleukemia virus type I. Proc Natl Acad Sci
USA 82.8359-8363
18. Kunitada S, Masako T, Tsoshiyuki T, Masanao M (1986) Requirement
ofmultiple copies of a 21-nucleotide sequence in the U3 regions
of human T -cell leukemia virus type land type Illong terminal repeats
for trans-acting activation of transcription. Proc Natl Acad Sci
USA 83.8112-8116
19. Miyoshi I (1981) Nature 294:770-774
20. Nagashima K, Yoshida M, Seiki M (1986) A single specjes of pX
mRNA of human T -cell leukemia virus type 1 encodes trans-activator
p40x and two other phosphoproteins. J Virol 60:394-399
21. Poiesz B et al.(1981)Proc Natl Acad Sci USA 77:7415-7419
22. Popovic M et al. (1983) Proc Natl Acad Sci USA 80:5402-5406
23. Rjce NR, Stephens RM, Couez D, Deschamps J, Kettmann R, Burny
A, Gilden RV(1984) Virology 138.82-93
24. Rosen CA, Sodroski JG, Haseltine W A (1985) Proc Natl Acad Sci
82.6502-6506
25. Rosen CA, Sodroski JG, Willems L, Kettmann R, Campbell K, Zaya
R, Burny A, Haseltine W A (1986) The 3' region of bovine leukemia
virus genome encodes a trans-activator protein. EMBO5(10):2585-2589
26. Sagata N, Yasunaga T, Tsuzuku-Kawamura J, Ohishi K, Ogawa Y,
Ikawa Y (1985a) Complete nucleotide sequence of the genome of bovinc
leukemia virus. its evolutionary relationship to other retrovirus.
Proc Natl Acad Sci USA 82:677-681
27. Sagata N, Yasunaga T, Igawa Y (1985b) Two distinct polypeptides
may be translated from a single splice mRNA of the X genes of human
T cell leukemia and bovine leukemia virus. FEBS Lett 192.37-42
28. Seiki M ct al. (1983a) Proc Natl Acad Sci
USA 80.3618-3622
29. Seiki Met al. (1983b) Nature 309:640-642
30. Seiki M, Hikikoshi A, Taniguchi T, Yoshida M (1985) Science
228:1532-1534
31. Sejki M, Inoue J, Takeda T, Yoshida M (1986) Direct evidence
that p40x of human T cell leukemia vjrus type I is a trans-acting
transcriptional activator. EMBO 5(3).561-565
32. Shimotohno et al. (1984) Proc Natl Acad Sci USA 81.6657-6661
33. Shimotohno K, Takahashi Y, Shimizyi N, Golde DW, Chen ISY, Miwa
M, Sugimara T (1985) Complete nucleotide sequence ofan infectious
clone of human T -cellleukemia virus type II: an open reading frame
for the protease. Proc Natl Acad Sci USA 82.3101-3105
34. Slamon DJ, Shimotohno K, Cline MJ, Golde DW, Chen ISY (1984)
Science 226:61-65
35. Slamon DJ, Press MF, Souza LM, Murdock DC, Cline MJ, Golde DW,
Gasson JC, Chen ISY (1985) Science 228.1427-1430
36. Sodroski J, Rosen C, Wong-Staal F, Salahuddin SZ, Popovic M,
Arya S, Gallo RC, Haseltine WA (1985a) Science 227:171-173
37. Sodroski J, Rosen C, Goh WC, Haseltine W (1985b) Science 228:1430-1434
38. Wachsman W, Golde DW, Temple PA, Orr EC, Clark SC, Chen ISY
(1985) Science 228:1534-1537
39. Watanabe T, Seiki M, Tsujimoto H, Miyoshi I, Hayami M, Yoshida
M (1985) Scquence homology of the simian retrovirus genome with
human T -cell leukemia virus type I. Virology 114:59-65
40. Yamamoto Met al. (1982) Science 217:737-740
41. Yoshinaka Y, Oroszlan S (1985) Bovine leukemia virus post-envelope
gene coded protein: evidence for expression in natural infection.
Biochem Biophys Res Com 131:347-354
|


