|
Clinical Epidemiology Branch, A 521 Landow Building. National Cancer Institute, Bethesda, Maryland 20014, USA Demography In studying etiology it is important to learn not only who is most susceptible but also who is least susceptible to certain forms of leukemia. Chronic lymphocytic leukemia (CLL) has a peculiarly low frequency in Chinese and Japanese. and is not induced by ionizing radiation [21]. CLL comprises only 1.5% of adult leukemias in Chinese and Japanese as compared with 30% in Europeans [ 10], and the frequency does not rise with migration to Hawaii [9]. These observations separate CLL etiologically from other forms of leukemia and indicate the extent to which inherent susceptibility may vary. Acute lymphocytic leukemia (ALL) in childhood has a peak frequency at about 4 years in white children, but not in Blacks, The peak emerged in Great Britain in the 1920's, in the U.S. in the 1940's and in Japan in the 1960's [24], but not yet in the People's Republic of China [25]. Thus ALL appears to be related to "industrialization" or development of the nation, but Blacks seem not to be susceptible. In the United States the peak rose progressively until the late 1950's, when a decline set in before the era of chemotherapy (Fig. I). Since 1972 the decline has steepened as new treatments have taken hold. Studies of cell surface markers indicate that the peak is due to ALL of the non-B non- T cell type [29].
Ionizing radiation: X-ray was the first environmental agent implicated
in leukemogenesis. Case reports in the 1920's were followed by retrospective
studies in the 1940's and a prospective study of atomic-bomb survivors
in Japan since the 1950's [21]. Myelogenous leukemia, either acute
or chronic, is the predominant type induced by radiation, but ALL
was occasionally induced, usually when the age at exposure was under
20 years. An increased frequency of CML persisted until 1965, but
the rates for acute leukemia continue to be elevated [4,16]. Recently
leukemia has been observed as a complication of radiotherapy for
Wilms' tumor [28], indicating the need to seek a lower dose that
would cure the original cancer without causing leukemia. Emphasis
is now being placed on the leukemogenic potential of lowdose radiation
in the general population. The question is more likely to be resolved
by understanding the biologic mechanisms involved than by arguments
about threshold and linearity of dose-response at levels below which
epidemiologic studies are impractical because of the large sample
sizes required. Beginning in 1956 Dr. Alice Stewart published data
which seemed to indicate that each form of childhood cancer could
be induced by small diagnostic x-ray exposures of the mother during
pregnancy. The fullest presentation of her findings appeared in
1975 [3]. One by one she had dealt with the criticisms of her original
study. The largest remaining puzzle was the constancy of the increase
in relative risk ( 1.5-fold ), regardless of the form of cancer.
be it leukemia, lymphoma. Wilms' tumor, cerebral tumors, neuroblastoma
or all other childhood cancer [2 I]. This finding seemed biologically
implausible [21]. Recent studies have failed to duplicate her findings
except possibly for the increased risk of leukemia [8,15,17]. A
lingering doubt thus remains about the interpretation o fher results.
Chemicals: Among chemicals known or suspected to be leukemogenic
are benzene in persons occupationally exposed [1,31], and alkylating
agents used for cancer chemotherapy [5]. Maternal exposure during
pregnancy could conceivably induce leukemia in the offspring transplacentally.
This possibility seems enhanced by a Swedish report that after occupational
benzene exposure during pregnancy, an increased frequency of sister
chromatid exchanges was observed in both mother and child [12] -a
finding which needs to be confirmed elsewhere. 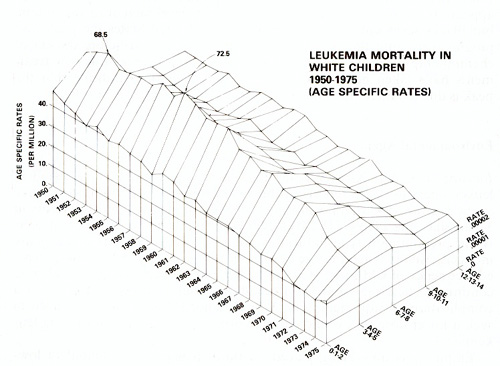
In the treatment of multiple myeloma with melphalan, the predominant subtype of leukemia induced has been acute monomyelogenous (AMML) [ 19]. Leukemia Clusters: In the mid-1960's leukemia clusters were taken by virologists as evidence for horizontal transmission of the disease. Creative statisticians were stimulated to develop dispassionate procedures to determine if clustering of such rare events in time and space was attributable to chance. When applied to leukemia, these methods showed no striking excess of clusters suggestive of an infectious mode of transmission [6]. Individual clusters may nonetheless be environmentally induced, as by ionizing radiation in Hiroshima and Nagasaki [16], and by benzene in Italian shoemakers [31 ]. Clusters are more likely to be meaningful if they are of a particular subtype as in Ankara, Turkey, where AMML accounted for 40% of childhood leukemia [7] as contrasted with 4% in Boston [ 11 ].
Inborn Chromosomal Abnormalities: It is now well known that leukemia, principally ALL. occurs excessively in Down's syndrome, but it is not well known that the childhood peak occurs three years earlier than in the general population [22]. An increased risk of leukemia might also exist in other autosomal trisomies, but may be less apparent because of the short lifespan. The risk of leukemia is markedly elevated in two recessively transmitted chromosomal fragility disorders, Bloom's syndrome [13] and Fanconi's anemia [26]. In Fanconi's anemia the cell type has almost invariably been AMML. It should be noted that this rare form of leukemia is the predominant form that occurs not only in this syndrome, but also after multiple myeloma treated with melphalan, and as a cluster in Ankara. In ataxia-telangiectasia (AT) there is both chromosomal fragility and immunodeficiency, and a predisposition especially to lymphoma, but also to ALL [14]. Each of the foregoing constitutional disorders, as well as exposure to ionizing radiation or benzene, has as a feature in common chromosomal abnormality before the onset of leukemia [23]. With the development of banding techniq ues for the examination of chromosomes, leukemia in the general population is increasingly being associated with particular chromosomal aberrations (Rowley, this volume). The piecing together of clinical and epidemiological rarities has thus led to a more broadly applicable understanding of cytogenetics of leukemia in the general population. It now becomes a challenge to explain the exceptions in which no connection is yet known between leukemia and chromosomal abnormality. Among inborn diseases with a predisposition to leukemia but as yet without characteristic chromosomal abnormalities are Poland syndrome [32]. Rubinstein- Taybi syndrome [ 18] and multiple neurofibromatosis, in which childhood leukemia is of the non-lymphocytic type [2]. A DNA Repair Defect in Familial AML? The discovery of DNA repair defects in xeroderma pigmentosum and AT, two disorders with acute sensitivity to radiant energy, led us to seek such defects in disorders with sensitivity to a delayed effect of radiation; namely, neoplasia. An extension of this reasoning led us to studies of persons with multiple primary cancers or familial cancer of types that could be radiogenic, but in these cases were not. In one instance a boy with hereditary retinoblastoma and multicentric osteosarcoma of the limbs, not due to therapy. showed diminished survival of skin fibroblasts in culture after x-irradiation. Another such case is under study. Study was also made of a family in which four siblings and three maternal relatives had acute myelogenous leukemia. and two other maternal relatives had malignant reticuloendotheliosis [30]. The occurrence of AML in the most recent sibling affected seemed to be predicted by increased transformation of skin fibroblasts in culture by SV40 seven years before the onset of leukemia [20]. This response was similar to that seen in Fanconi's anemia (FA) or in heterozygotes for the disease. but no stigmata of FA was observed in the family. The available skin fibroblasts, from two of the affected siblings and the mother, showed diminished cell survival, but those from healthy twin brothers and the father did not [27]. The cells are now being studied for DNA repair defects. These observations illustrate once again the importance of an interaction among epidemiology, clinical observations and laboratory research.
1 Aksoy. M Benzene and Ieukaemia. Lincet 1978 1,441 2 Badcr. J L. Miller. R W. Neurofibromatosis and childhood leukcmia J Pcdia tr 92, 925-929 ( 1978 ) 3 Bithell. J F. Stewart. AM Prenatal irradiation and childhood malignancy A rcvicw of British data from the Oxford survey Br J Cancer 31,271 287 ( 1975) 4 Bizzozero 01. Jr. Johnson. KG. Ciocco. A. Radiation-related Ieukemia in Hiroshima and Nagasaki. 1946-1964 I Distribution. incidence and appearance time N Engl J Med 274, 1095-1101 (1966) 5. Bloomfield. C D. Brunning. R D. Acute Ieukemia as a terminal event in nonleukemic hematopoietic disorders Semin Oncology 3,297-313 ( 1976 ) 6 Caldwell. G G. Heath- C W. Jr Casc clustering in cancer South Med J 69, 15981602 ( 1976) 7 Cavdar. A 0. Arcasoy- A- Babacan E G÷zdaoglu. S. Topuz. ▄. Fraumni. J I-. Jr Ocular granulocytic sarcoma (chloroma) with acute myelomoncvtic leukemia in Turkish children Cancer41, !606-1609 (1978) 8 Diamond. E. L. Schmerler. H. Lilienfeld. AM The relationship of intra-uterine radiation to subsequent mortality and development of leukemia in children A prospective study. Am J Epidemiol97, 283-313 (1973) 9. Elizaga. F V. Oishi. N Chronic lymphocytic leukemia in Japanese in Hawaii Hawaii Med 1.36, 169-17! (1977) 10 Finch. SC. Hoshino.T. Itoga.T.. Ichimaru.M Ingram.RH..Jr. Chronic lymphocytic leukemia in Hiroshima and Nagasaki. Japan Blood 33,79-86 ( 1969) II Fraumeni. J F. Jr. Manning. M D. Mitus. WJ. Acute childhood leukemia. Epidemiologic study by cell type of 1263 cases at the Children's Cancer Research Foundation in Bostom. 1947-65 J. Nat! Cancer Insl46, 461-470 (1971) 12 Funes-Cravioto. ~. Zapata-CGayon. C. Kolmodin-Hedman. B. Lambert. B. Lindsten.1. Norberg. E. Nordenskj÷ld. M. Olin. R. Swensson. A Chromosome aberrations and sister-chromatid exchange in workers in chemical laboratories and a rotoprinting factory and in children of women laboratory workers Lancct 1977 II, 322-325 13. German. 1. Bloom. D. Passarge. E. Bloom's syndrome V Surveillance for cancer in affected families. Clin Genelli 162-168 (1977) 14 Hechl F. McCaw. B K. Koler. R D. Ataxia-telangiectasiation Iymphocytes N Engl 1 Med 289, 286-291 ( 1973 ) 15 Hutchison. GB. Late neoplastic changes following medical irradiation Cancer 37, 1102 1107 ( 1976) 16 Ichimaru. M. Lshimaru. T. Leukemia and related disorders 1 Radiat Res 16, 89-96 (1975) 17 Jablon, S. Kato. H. Childhood cancer in relation to prenatal exposure to atomic-bomb radiation Lancet 1970 II, 1000-1003 18 Jonas, D. M.. Heilbron, D. C., Ablin. A. R.. Rubinstein -Taybi syndrome and acute leukemia 1.Pediatr.92,851-852(1978) 19. Karchmer, R K, Amare, M., Larsen, W. E., Mallouk, A G, Caldwell, G. G.. Alkylating agents as leukemogens in multiple myeloma. Cancer 33, L103-1107 ( 1974) 20 McKeen, E. A., Miller, R W., Mulvihill, 1.1., Blattner, W A, Levine, AS Familial leukaemia and SV40 transformation. Lancet 1977II, 310 21 Miller, R. W.. Delayed radiation effects in atomic-bomb survivors Science 166,569-574 (1969) 22 Miller, R.W. Neoplasia and Down's syndrome. Ann NY Acad Sci 171,637-644 (1970) 23. Miller, R W.. The feature in common among persons at high risk of leukemia In. Biology of Radiation Carcinogenesis. Yuhas. 1. M.. Tennanl R. W. Regan, 1 D. (eds). pp 45-50 New York Raven Prcss 1976 24 Miller, R W Ethnic differences in cancer occurrence. Genetic and environmcntal influences with particular rcference to neuroblastoma In CGenetics of Human Cancer Mulvihill, 11, Miller, R W. Fraumeni, 1F. Jr (eds). pp I- 14 New York Raven Press 1977 25 Miller, R. W Cancer epidemics in the People's Republic of China 1 Natl Cancer Lnsl 60, 1195-1203 (1978) 26 O'Gorman Hughes, D.W.. Aplastic anaemia in childhood LIL Constitutional aplastic anaemia and related cytopenias Med.1 Ausl 1,519-526 ( 1974 ) 27 Paterson, MC Unpublished data 28. Schwartz, A D., Lee, H, Baum. E S. Leukemia in children with Wilms tumor. 1 Pediatr 87,374-376 (1975) 29 Sen. L, Borella, L. Clinical importance of Iymphoblasts with T markers in childhood acute leukemia N Engl 1 Med 292,828-832 (1975) 30 Snyder, AL, Li, F.P, Henderson, ES. Todaro, G1 Possible inherited leukemogenic factors in familial acute myelogenous leukemia Lancet 1970 1,586-589 v 31 Vigliani. EC., Forni, A.. Benzene and leukemia :nviron Res 11, 122-L27 (1976) 32 Waters, T. R.. Reddy. B. N. Bailon. A. Vitale. L F. Poland's syndrome associated with leukemia 1. Pediatr:82, 899 (1973) |