|
A. Abstract Human lymphocyte subpopulations as well as leukemic lymphocytes can be identified and enumerated in blood smears by using bacteria that bind spontaneously to lymphocytes or by using bacteria to which antibodies are chemically coupled. The mechanism of natural binding of bacteria to lymphocytes was shown to involve a lectin on the lymphocyte surface and a carbohydrate on the bacteria. Also, we found that natural killer (NK) cells can be separated by negative selection using monolayers of bacteria. A subpopulation of T cells, identified by their binding of B. globigii, was shown to be suppressors for NK cells. B. Introduction The methods used routinely to identify lymphocyte subpopulations involve the separation of lymphocytes from other blood cells followed by staining with fluorescent antibodies and/or rosette formation with erythrocytes. These procedures are all difficult to standardize and suffer from subjective interpretations. Moreover, the loss of particular subpopulations of cells and the inability to assess the cellular morphology can cause inaccuracies. We have developed methods of identifying lymphocyte subpopulations in blood smears by using bacteria as carriers for purified antibody against cell membrane antigens or bacteria that bind spontaneously to lymphocytes (Teodorescu et al. 1977a, 1979a). Antibody-coated bacteria have been used to identify Band T cells in smears of peripheral blood and bacteria that bind spontaneously have been used to identify and enumerate B cells as well as two Band four T cell subpopulations. Also, bacteria have been used to identify leukemic lymphocytes in cell suspensions or in blood smears (Nelson et al. 1979; Teodorescu et al. 1977b). A method has been developed to separate various lymphocyte subpopulations by bacterial adherence and functional differences among them have been demonstrated (Kleinman and Teodorescu 1978, 1979 ; Kleinman et al. 1980).
I. Bacterial suspensions Bacteria were grown and fixed as previously described (Teodorescu et al. 1979a). II. Labeling the Lymphocytes with Bacteria in Stained Blood or Bone Marrow Smears The procedure previously described has been followed. Briefly, heparinized blood was collected and the cells were washed. Bacteria were added in excess to small samples of blood cells, centrifuged for 6 min at 900 9 to promote binding, and centrifuged twice more at 150 9 for 10 min to remove the unbound bacteria. The suspension was smeared and stained with Wright's stain. III. Separation of Lymphocyte Subpopulations by Bacterial Adherence The procedure previously described was foIlowed (Kleinman and Teodorescu
1978, 1979; Kleinman et al. 1980). Briefly, bacteria were coupled
to glutaraldehyde-fixed gelatin layers. Monocytes were removed by
glass wool adherence and the lymphocytes purified by Ficoll-hypaque
gradient centrifugation. The lymphocytes were centrifuged against
bacterial monolayers, and the nonadherent ceIls were separated from
the adherent cells. D. Results and Discussion I. Acute Lymphocytic Leukemias With rare exceptions, bacteria bound abundantly to lymphocytes
(Fig. 1). We studied 12 patients with acute lymphocytic leukemia)
(ALL) using bacteria as well as fluorescent antibodies (Hsu and
Morgan 1980). Of these cases five were classified as pre-E cells
based on a relatively low percentage of Ig+ cells but high percentage
of lymphocytes binding E melitensis, a E cell marker independent
of surface Ig (Teodorescu et al. 1979b) .In all five of these patients,
although the percent age of Ig- Em + cells was high, the percentage
of IgT cells was relatively low with a relatively normal epsiloni
gamma ratio (Fig. 2). This observation suggests that the cells were
arrested at a stage of differentiation much earlier than that in
which the surface Ig is exposed. In chronic lymphocytic leukemia
(CLL) it appears that cells with undetectable surface Ig coexist
with Ig-bearing cells of only one type of light chains (Nelson et
al. 1979). The existence in one patient of a higher percentage of
Ig+ cells than cells that bound B. melitensis suggests that sometimes
the Ig is of exogenous origin. This was also reflected by the large
overlap between epsilon-bearing and gammabearing lymphocytes. 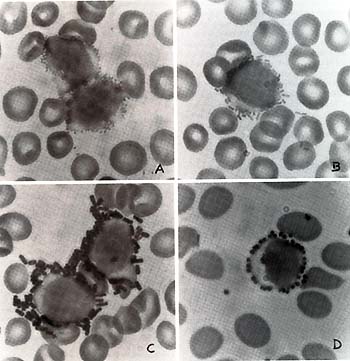 Fig. 1. Lymphocytes labeled by bacteria in blood smears of patients with leukemia. A B. melitensis,. B E. coli,; C B. globigii and D S. aureus
One patient (14-month-old female) had familial chronic myelocytic leukemia (four cases diagnosed in the same family, Ph 1- ) .The patient was studied here during an excerbation and found to have relatively high percentage of Bill+ lymphocytes which was much higher in the peripheral blood than in the bone marrow. At the same time the percentage Ig + cells was normal. The coexistence of leukemic pre-B cells with CML cells was also described in 3 out of 20 cases of CML by Greaves (Greaves 1979). This observation suggests that B cells and myelocytes may have an immediate common precursor . II. The Mechanism of Binding of Bacteria by Lymphocytes We put forward the hypothesis that bacteria bind as the result of an interaction between a lectin on the lymphocyte surface and a carbohydrate on the bacteria (Teodorescu et al. I979b ). The following results were obtained in its support : I. The binding of B. melitensis to B cells was prevented by alfa-methyl-D-mannoside ( a-MM) but not by other sugars, suggesting that one of the lectins involved in binding is similar to Concanavalin A (Con A) ; 2. The binding of B. melitensis to B cells was prevented by pretreatment of the peripheral blood lymphocytes (PBL) with 5% alfa-MM, but pretreatment of bacteria had no effect ; 3. An Escherichia coli mutant (strain 2023) which binds to B cells and part of the T cells was also agglutinated by Con A, but its parental strain was not; the binding of this mutant to B cells was also inhibited by a-MM; 4. Bacteria that bind to human lymphocytes were agglutinated at high titers by various plant lectins, while those that do not bind were agglutinated at low titers or not at all ; 5. Bacteria that bind to B-cells as well as those that bind to B- and T -cells were agglutinated by Con A, Lens culinaris agglutinin, and Pisum sativurn agglutinin, whose carbohydrate specificities were alfa-D-mannosyl- and alfa-D-glucosyl- residues ; 6. The "receptors" on lymphocytes but not those on bacteria were sensitive to pronase, suggesting that the protein (lectin) was on the lymphocyte surface; and 7. Bacteria still bound after being heated at 121°C or being fixed with formaldehyde. Lectin-sugar interactions have been shown to be involved in a variety of cellular interactions and recognition processes (Simpson et al. 1978). Since lymphocyte subpopulations are selectively responsive to different lectins, these cells may interact among themselves or with other cells using their lectins or their carbohydrates. Thus, bacteria may recognize functional "arms" of lymphocyte subpopulations.
Both E. coli coated with anti-light chain antibody and B. melitensis bind to a substantial number of CLL lymphocytes (Nelson et al. 1979; Teodorescu et al. 1977b) .Other bacteria have also been found to bind to these cells, suggesting the existence of a heterogenity within the malignant clone (Teodorescu et al. 1977b ). Based on our results suggesting that lymphocytes have surface lectins, we speculated that these lectins are somehow involved in the control by other cells of malignant lymphocyte proliferation. Therefore, we put forward the hypothesis that with the progression of disease lymphocytes with less lectins are selected and grow uncontrolled. We determined the binding of several strains of bacteria to CLL lymphocytes in blood smears of 24 patients. We found a statistically significant correlation (p=O.O01) between binding indices and symptom status, i.e., the symptomatic patients had an average binding index of 35% and the asymptomatic 56.6% (Nelson et al. 1979). To demonstrate whether our observation is also relevant in predicting patient survival, we listed 12 patients in the order of binding indexes (Table 1) and followed them longitudinally. We found that the patients with low binding index also had poor survival rates, suggesting that this index may be of prognostic value. Table 1. The relationship between binding
indices for bacteria and survival in CLL patients a 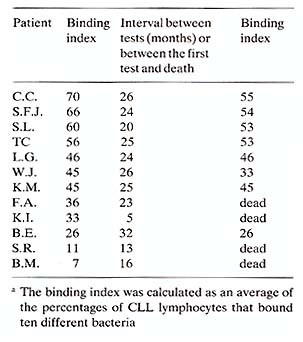
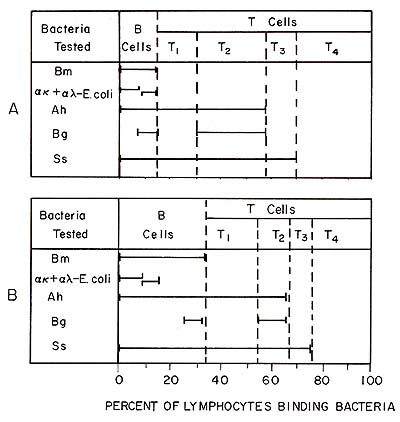
We have previously shown that some of the lymphocyte subpopulations identified by bacterial adherence are functionally different (Kleinman and Teodorescu 1978; 1979 ; Kleinmann et al. 1980). Since T 4 cells do not bind any bacteria, they were readily isolated by negative selection by adsorbing on bacterial monolayers B cells, T1, T 2, and T 3 cells. Most of the natural killer (NK) activity of the peripheral blood lymphocytes (PBL) was concentrated in the T 4 lymphocyte subpopulation (Kleinman et al. 1980). The T 4 cell population contained about 75% cells with receptors for Fc of IgG, which have been shown to be indicative of NK cells (West et al. 1977). We investigated whether the activity of NK cells was controlled by another lymphocyte subpopulation identified by bacteria. When the 51Cr release in 4-h assay was determined at increasing ratios of lymphocyte/target cells, we found that the PBL and T 4 cell curves never merge. This observation suggested that the T 4 cells were prevented from acting by another cell population. This inhibitory effect was not due to a simple dilution of NK cells, steric interference, or to a competition for targets. In fact, only when living T 2 cells were added to T 4 cells did the inhibition occur; when T 1 T 3 cells were added the inhibition did not occur. Thus, T 2 cells appear to be suppressors for NK cells. Although evidence has been accumulating that the NK activity is important in vivo in the defense against leukemic cell proliferation, the reason for the exquisite sensitivity of malignant cells to NK cells has not been demonstrated. It is worth noting that T 4 cells do not bind any of the bacteria tested ( over 60 strains tested), and therefore, they are unlikely to have lectins. On the other hand, lymphoblastoid cell lines bind well and indiscriminately various bacteria. Thus, we may speculate that during malignant transformation various new lectins are exposed and attract preferentially the "Iectinless" (negative replica) lymphocytes (or monocytes), resulting in killing. When we tested the binding properties of T 4 cells compared with T 2 cells, we found that the former binds exclusively to CEM Iymphoblastoid cells but not to Chang hepatoma cells and that T 2 cells bound well to Chang cells but not to CEM cells. Based on the results presented above or published elsewhere (Nelson et al. 1979) bacteria can be useful reagents for the identification and characterization of leukemic lymphocytes and of the cells that may be involved in the defense against leukemic cells. Since we have developed the necessary technology of selecting mutants of E. coli with various binding properties (Mayer and Teodorescu, 1980), the possibility exists of developing a large number of useful reagents that offer obvious advantages.
-Greaves MF (1979) In: Neth R, Gallo RG, Hofschneider PH, Mannweiler
K ( eds) Modern trends in human leukemia III, Springer, Berlin Heidelberg
New York, p 335 |