|
1 Immunex Carp., 51 University St., Seattle, WA 98101, USA.
During the past few years it has become clear that many membrane proteins can be found in soluble forms in body fluids. Examples include histocompatibility antigens [1] and Fc receptors [2]. The extracellular portion of the membrane protein may be released by proteolysis, or by phospholipase action in the case of those molecules that are linked to the membrane by a phosphoinositollinkage. Alternatively, the soluble protein may be encoded by an alternatively spliced messenger ribonucleic acid (mRNA) species. Frequently the soluble, extracellular portion of the protein retains the same ligand-binding properties as the membrane-bound form. Cytokine receptors also show the same behavior. Soluble receptors that retain ligand-binding properties have been found in urine and serum for the interleukin-2 (IL-2) receptor-alpha [3], tumor necrosis factor (TNF) receptor (two forms) [4], IL-6 receptor [5], gammainterferon (gamma-IFN) receptor [5], growth hormone receptor [6], and nerve growth factor receptor [7]. Recently we have identified alternatively spliced mRNA species that encode soluble forms of the IL-4 and IL- 7 receptors [8, 9]. Here we discuss these results and describe how soluble cytokine receptors, either naturally occurring forms or generated by recombinant deoxyribonucleic acid (DNA) manipulation, can be used as immunomodulatory agents, both in vitro or inn VIVO. Cytokine Receptor Families Elucidation of the primary amino acid sequences of many cytokine
receptors as a result ofcDNA cloning has allowed the grouping of
these receptors into families, based on similarities in their extracellular
, ligand-binding domains. For those receptors whose ligands regulate
hematopoiesis and immunity, three families have emerged. The first
family is the wellknown and very large immunoglobulin superfamily,
most of whose members are not cy to kine receptors. However, the
receptors for IL-1, colony stimulating factor-1 (CSF-1), and PDGF
belong to this group, with three, five, and five immunoglobulin-like
domains respectively in their extracellular portions [10, 11]. CSF-1
and PDGF receptors have intracellular tyrosine kinase domains, the
IL-1 receptor does not. The second, more recently recognized, family
consists solely of cytokine receptors. We have designated this as
the hematopoietin receptor family as almost all of these receptors
mediate effects on hematopoietic cells [12]. The members of this
family currently consist of the receptors for IL-2 (ß subunit) [13,
14], IL-3 [15], IL-4 [8, 12], IL-6 [16, 17], IL- 7 [9], GM-CSF [18,
19], G-CSF [20], erythropoietin [21], prolactin (two forms of receptor)
[22, 23], and growth hormone [6]. The common sequence element in
the extracellular domains of these receptors is a stretch of about
200 amino acids that shows considerable sequence conservation between
the different members of the family [8, 18, 24, 25]. When these
sequences are compared using the ALIGN program to generate pairwise
scores that measure the degree of amino acid sequence relatedness,
scores are mostly in the range of 3-12 [24]. Any score greater than
3 is considered to indicate significant sequence relatedness [10].
Within these 200 amino acids there are certain features that show
particular conservation (see Fig.1). These include the positions
of four N-terminal cysteines (although many family members have
additional nonconserved cysteines) and a WSXWS motif located at
the C-terminus of the conserved region, usually just outside the
transmembrane domain. The Cterminal 90-100 amino acids of the con
served region show significant homology to type III fibronectin
domains [26], and the G-CSF receptor is so far unique in having
three additional fibronectin-like domains between the conserved
region and the transmembrane domain [19,20]. It can be speculated
that the fibronectinlike domains playa role in interaction of the
growth factor receptors with extracellular matrix components or
other cell surface proteins. The IL-3 receptor has a duplication
of the 200 amino acid conserved region [15], and the IL-6 and G-CSF
receptors have N-terminal immunoglobulin-like domains [16, 19, 20],
showing that receptors can belong to more than one family. 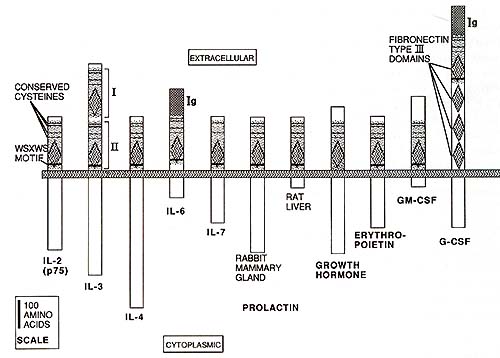
In contrast to the striking degree of sequence relatedness between the extracellular domains of the receptors, the cytoplasmic sequences show little if any similarity apart from a general tendency towards a high content of serine, proline, and acidic amino acids. This reflects our current ignorance as to the mechanisms of signal transduction by these receptors. The third family centers around the newly cloned TNF receptors p 80 and p 60 [27 -29]. Both these molecules have a cysteine-rich, extracellular, ligand-binding domain that can be subdivided into four internally homologous subdomains. Other members of the family sharing this structure are the nerve growth factor receptor [30]. CD4O, a cell surface protein involved in B cell activation [31], 4-1 BB, characterized as a mRN A species induced upon T cell activation [32], and OX40, a membrane protein present on rat CD4+ T cells that can contribute to T cell proliferation [33]. The last three proteins may well be cytokine receptors with unknown ligands. An additional member of this family, with particularly strong homology to TNF receptor p 80, is the T 2 open reading frame from Shope fibroma virus, a rabbit pox virus [34]. The predicted protein sequence has characteristics of a secreted TNF receptor, and we have shown that the T2 ORF can be expressed in mammalian cells. The protein is secreted and binds TNF [35]. It seems likely that the virus has acquired a rabbit TNF receptor during evolution and that it expresses a soluble TNF receptor as a defense against the portion of the host's immune response mediated by TNF. Once again, the members of the TNF receptor family show little or no sequence relatedness in their cytoplasmic domains, nor do the IL-1 and TNF receptors, despite the fact that IL-1 and TNF share many biological activities.
The existence of soluble extracellular domains of cytokine receptors that retain their ligand-binding capabilities suggested that such molecules might be able to block interaction of their cognate ligands with cell surface receptors. This might have a normal immunoregulatory role in vivo, or could be exploited pharmacologically to down-modulate undesirable immune reactions, such as allergy, autoimmunity, or graft rejection. In order to test this hypothesis, we have expressed soluble murine IL-1 and IL-4 receptors in mammalian cells and purified the recombinant proteins by affinity chromatography. The soluble IL-1 receptor was generated by inserting a translation termination codon immediately 5' to the transmembrane domain [36], and the soluble IL-4 receptor used a cDNA from a naturally occurring, alternatively spliced mRNA species [8]. The purified receptors were tested for their ability to block specifically the biological activities of their respective ligands. IL-l and IL-4 can each stimulate B cell proliferation when anti-immunoglobulin is used as a co-mitogen. IL-l mediated B cell proliferation was completely inhibited by soluble IL-1 receptor, whereas soluble IL-4 receptor had no effect. Conversely, IL-4 mediated B cell proliferation was inhibited by soluble IL-4 receptor but not by soluble IL-l receptor [37]. These results demonstrate not only that soluble IL-l and IL-4 receptors have highly specific neutralizing capacity, but also that IL-l and IL-4 mediate B cell proliferation by independent pathways. Following the demonstration of in vitro biological activity, the soluble receptors were tested in vivo in two models that involve lymphocyte activation in response to alloantigenic challenge [38, 39]. In the first, Balb/c mice were injected in the footpad with irradiated allogeneic spleen cells from C 57 BL/6 mice. Over the course of 7 days there was a hostversus-graft response leading to lymphoproliferation and consequent swelling of the draining popliteal lymph nodes. The strength of this reaction could be quantitated by excision and weighing of the lymph nodes. As a control, each mouse was injected in the contralateral footpad with an equal number of syngeneic, irradiated spleen cells, so that the specific response could be measured as the weight of the lymph nodes draining the site of allogeneic cell injection minus the weight of the lymph nodes draining the site of syngeneic spleen cell injection. Daily injections of soluble IL-1 receptor or soluble IL-4 receptor could completely block the lymphoproliferative response. Injections were given intraperitoneally or subcutaneously for 4 days, using mouse serum albumin as a negative control. As little as 100 ng -1 µg per dose of receptor showed significant inhibition, and the optimum time to commence treatment was 1 day prior to challenge with the allogeneic spleen cells [38, 39]. In each case, the inhibitory effect of the soluble receptor could be reversed by its cognate ligand. In a second model system, hearts from newborn C 57 BL/6 mice were grafted into ear pinnae of Balb/c mice. The hearts continued to beat until rejected by the hosts at around 12 days after transplantation. Daily administration of soluble IL-1 receptor or soluble IL-4 receptor for 4-6 days, starting on the day oftransplantation, significantly prolonged graft survival [38, 39]. These data implicate both IL-1 and IL4 as being important in the initiation of an immune response to alloantigenic challenge in vivo, and suggest that both soluble receptors may be clinically useful in preventing graft rejection. Based on the known biological activities of IL-1 and IL-4, it might be predicted that the soluble receptors would be of therapeutic value in other disease states. IL-1 has many pro-inflammatory properties; examples include induction of prostaglandin release, stimulation of cartilage breakdown, and induction of cytokines with chemotactic activity for neutrophils and monocytes. Soluble IL-1 receptor might be a useful anti-inflammatory agent in diseases such as rheumatoid arthritis. IL-4 promotes synthesis of IgE by an isotype class-switching mechanism in B cells and is a growth factor for mast cells in conjunction with IL-3. It is thought to be a central mediator of allergic responses and consequently soluble IL-4 receptor may have therapeutic value in controlling allergy. The demonstrated efficacy of soluble cy to kine receptors as immunomodulators opens up possibilities for clinical intervention in many disease states, and this promises to be an area of active investigation.
I thank Judy Reaveley for preparation of the manuscript, and many colleagues at Immunex for discussions and contributions of information, especially Carl March, Pat Beckmann, Craig Smith, Bill Fanslow, Charlie Maliszewski, Mike Widmer, Ray Goodwin, Linda Park, Alf Larsen, Bruce Mosley, Rejean Idzerda, John Sims and Steve Dower.
1. Kress M, Cosman D, Khoury a, Jay a (1983) Secretion of a transplantationrelated antigen. Cell 34: 189 2. Pure E, Durie CJ, Summerill CK, Unkeless JC (1984) Identification of soluble Fc receptors in mouse serum and the conditioned medium or stimulated B cells. J Exp Med 160:1836 3. Marcon L, Fritz ME, Kurman CC, Jensen JC, Nelson DL (1988) Soluble Tac peptide is present in the urine of normal individuals and at elevated levels in patients with adult T cell leukaemia (A TL). Clin Exp Immunol 73: 29 4. Engelmann H, Novick D, Wallach D (1990) Two tumor necrosis factor-binding proteins purified from human urine. J BioI Chern 265:1531 5. Novick D, Engelmann H, Wallach D, Rubinstein M (1989) Soluble cytokine receptors are present in normal human urine. J Exp Med 170: 1409 6. Leung DW, Spencer SA, Cachianes a, Hammonds Ra, Collins C, Henzel WJ, Barnard R, Waters MJ, Wood WI (1987) Growth hormone receptor and serum binding protein: purification, cloning and expression. Nature 330: 537 7. Zupan AA, Osborne PA, Smith CE, Siegel NR, Leimgruber RM, Johnson EM Jr ( 1989) Identification, purification, and characterization of truncated forms of the human nerve growth factor receptor. J BioI Chell 264:11714 8. Mosley B, Beckmann MP, March CJ, Idzerda RL, Gillpel SD, VandenBos T, Friend D, Alpert A, Anderson D, Jackson J, Wignall JM, Smith C, Gallis B, Sills JE, Urdal D, Widmer MB, Cosman D, Park LS (1989) The murine interleukin-4 receptor: molecular cloning and characterization of secreted and membrane bound forms. Cell 59: 335 9. Goodwin RG, Friend D, Ziegler SF, Jerzy R, Falk BA, Gillpel S, Cosman D, Dower SK, March CJ, Nallen AE, Park LS (1990) Cloning of the human and murine interleukin- 7 receptors: demonstration of a soluble form and homology to a new receptor superfallily. Cell 60:941 10. Williams AF, Barclay AN (1988) The immunoglobulin superfallily -domains for cell surface recognition. Annu Rev Illmunol6:381 11. Sills JE, March CJ, Cosman D, Widmer MB, MacDonald HR, McMahan CJ, Grubin CE, Wignall JM, Jackson JL, Call SM, Friend D, Alpert AR, Gillis S, Urdal DL, Dower SK (1988) cDNA expression cloning of the IL-l receptor, a member of the immunoglobulin superfallily. Science 241:585 12. Idzerda RL, March CJ, Mosley B, Lyman SD, VandenBos T, Gillpel SD, Din WS, Grabstein KH, Widmer MB, Park LS, Cosman D, Beckmann MP (1990) Human interleukin 4 receptor confers biological responsiveness and defines a novel receptor superfallily. J Exp Med 171: 861 13. Hatakeyama M, Tsudo M, Minalloto S, Kono T, Doi T, Miyata T, Miyasaka M, Taniguchi T (1989) Interleukin 2 receptor ß chain gene: Generation of three receptor forms by cloned human alfa and ß chain cDNA's. Science 244:551 14. Kono T, Doi T, Yallada G, Hatakeyama M, Minalloto S, Tsudo M, Miyasaka M, Miyata T, Taniguchi T (1990) Murine interleukin 2 receptor ß chain: Dysregulated gene expression in lymphoma line EL-4 caused by a promoter insertion. Proc Natl Acad Sci USA 87:1806 15. Itoh N, Yonehara S, Schreurs J, Gorman DM, Maruyama K, Ishii A, Yahara I, Ken-Ichi Y, Miyajima A (1990) Cloning of an interleukin-3 receptor gene: A member of a distinct receptor gene family. Science 247:324 16. Yallasaki K, Taga T, Hirata Y, Yawata H, Kawanishi Y, Seed B, Taniguchi T, Hirano T, Kishilloto T (1988) Cloning and expression of the human interleukin-6 (BSF-2/IFNß2) receptor. Science 241: 825 17. Sugita T, Totsuka T, Saito M, Yallasaki K, Taga T, Hirano T, Kishilloto T (1990) Functional murine interleukin 6 receptor with the intracisternal a particle gene product at its cytoplasmic domain. J Exp Med 171:2001 18. Gearing DP, King JA, Gough NM, Nicola NA (1989) Expression cloning of a receptor for human granulocyte-llacrophage colony-stillulating factor. EMBO J 8 : 3667 19. Fukunaga R, Ishizaka-Ikeda E, Seto Y, Nagata S (1990) Expression cloning of a receptor for murine granulocyte colonystimulating factor. Cell 61: 341 20. Larsen A, Davis T, Curtis BM, Gillpel S, Sills JE, Cosman D, Park L, Sorensen E, March CJ, Smith CA (1990) Expression cloning of a human granulocyte colonystimulating factor receptor: a structural mosaic of hematopoietin receptor, immunoglobulin and fibronectin domains. J Exp Med 172:1559 21. D'Andrea AD, Lodish HF, Wong GG (1989) Expression cloning of the murine erythropoietin receptor. Cell 57: 277 22. Boutin J-M, Jolicoeur C, Okallura H, Gagnon J, Edery M, Shirota M, Banville D, Dusanter-Fourt I, Djiane J, Kelly PA (1988) Cloning and expression of the rat prolactin receptor, a member of the growth hormone/prolactin gene family. Cell 53: 69 23. Edery M, Jolicoeur C, Levi-Meyrueis L, Dusanter-Fourt I, Petridou B, Boutin JM, Lesueur L, Kelly PA, Djiane J (1989) Identification and sequence analysis of a second form of prolactin receptor by molecular cloning of complementary DNA from rabbit mammary gland. Proc Natl Acad Sci USA 86:2112 24. Cosman D, Lyman SD, Idzerda RL, Beckmann MP, Park LS, Goodwin RG, March CJ (1990) Anew cy to kine receptor superfamily. Trends Biochell Sci 15: 265 25. Bazan JF (1989) A novel family of growth factor receptors: a common binding domain in the growth hormone, prolacin, erythropoietin and IL-6 receptors, and the p75 IL-2 receptor ß chain. Biochell Biophys Res Commun 164: 788 26. Pat thy L (1990) Homology ofa domain of the growth hormone/prolactin receptor family with Type III modules of fibronectin. Cell 61:13 27. Smith CA, Davis T, Anderson D, Solam L, Beckmann MP, J erzy R, Dower SK, Cosman D, Goodwin RG (1990) A receptor for tumor necrosis factor defines an unusual family of cellular and viral proteins. Science 248:1019 28. Loetscher H, Pan y -C, Lahm H-W, Gentz R, Brockhaus M, Tabuchi H, Lesslauer W (1990) Molecular cloning and expression of the human 55 kd tumor necrosis factor receptor. Cell 61:351 29. Schall TJ, Lewis M, Koller KJ, Lee A, Rice GC, Wong GHW, Gatanaga T, Granger GA, Lentz R, Raab H, Kohr WJ , Goeddel DV (1990) Molecular cloning and expression of a receptor for human tumor necrosis factor. Cell 61: 361 30. Johnson D, Lanahan A, Buck CR, Sehgal A, Morgan C, Mercer E, Bothwell M, Chao M (1986) Expression and structure of the human NGP receptor. Cell 47: 545 31. Stamenkovic I, Clark EA, Seed B (1989) A B-lymphocyte activation molecule related to the nerve growth factor receptor and induced by cytokines in carcinomas. EMBO J 8: 1403 32. Kwon BS, Weissman SM (1989) cDNA sequences of two inducible T -cell genes. Proc Natl Acad Sci USA 86:1963 33. Mallett S, Possum S, Barclay AN (1990) Characterization of the MRC OX40 antigen of activated CD4 positive T lymphocytes -a molecule related to nerve growth factor receptor. EMBO J 9: 1063 34. Upton C, DeLange AM, McPadden G ( 1987) Tumorigenic poxviruses: Genomic organization and DNA sequence of the telomeric region of the Shope fibroma virus genome. Virology 160: 20 35. Smith CA, Davis T, Wignall JM, Din WS, Parrah T, Upton C, McPadden G, Goodwin RG (1991) T2 open reading frame from the Shope fibroma virus encodes a soluble form of the TNP receptor. Biochem Biophys Res Commun 176:335 36. Dower SK, Wignall JM, Schooley K, McMahan CJ, Jackson JL, Prickett KS, Lupton S, Cosman D, Sims JE (1989) Retention of ligand binding activity by the extracellular domain of the IL-l receptor . J Immunol142:4314 37. Maliszewski CR, Sato TA, VandenBos T, Waugh S, Dower SK, Slack J, Beckmann MP, Grabstein KH (1990) Cy to kine receptors and B cell functions I. Recombinant soluble receptors specifically inhibit IL-l and IL-l induced B cell activities in vitro. J Immunol144: 3028 38. Panslow WC, Sims JE, Sassenfeld H, Morrissey PJ, Gillis S, Dower SK, Widmer MB ( 1990) Regulation of alloreactivity in vivo by a soluble form of the interleukin-l receptor. Science 248: 739 39. Fanslow WC, Clifford KC, Park LS, Rubin AS, Voice RF, Beckmann MP, Widmer MB (1991) Regulation of alloreactivity in vivo by IL-4 and the soluble IL-4 receptor. J Immunol (in press) |