|
A. Introduction
A dominant paradigm of cancer research is that alterations in the
cell surface are of paramount importance to tumour cell behavior
(Wallach 1978; Marchesi 1976). It is widely held that this is in
part reflected in the regular expression of neo-antigens resulting
from gene derepression [ or "retrogressive differentiation" (Coggin
1978)], mutation (Baldwin 1974) or altered processing [ e.g. glycosylation
(Hakomori 1975)]. The search for novel antigens or other cell surface
features of human tumour cells has an obligatory control demand
which is frequently ignored or inadequately dealt with, i.e. that
the appropriate cellular controls be analysed in parallel. Since
most epithelial carcinomas and acute leukemias probably arise from
tissue stem cells and, moreover, frequently have a maturation arrest
imposed upon them, it should be selfevident that (a) many or most
of the consistent phenotypic features of leukemic cells (and tumour
cells in general) will be a reflection of their immature cell origins
and (b) the significance of potentially unique biochemical or molecular
features of tumour cells cannot be interpreted until we have access
to normally infrequent tissue precursor cells. The latter demand
may be satisfied in the future by the development of new culture
methods (see Dexter, this volume) ; in the meantime one of the most
incisive approaches we have to the analysis of tumour cell phenotypes
is the serological characterization of cell surface antigen expression
on individualleukemic cells, particularly by monoclonal antibodies.
The crucial advantages of leukemia in this context are that "equivalent"
normal tissue is available in a physical form that is amenable to
"cell surface" serology (i.e. single cell suspensions) and that
stem cells and progenitor cells, whilst not morphologically recognisable,
can be detected by functional assays in vitro. By the same token,
acute leukemias offer an opportunity to discover antigenic and other
characteristic marker features of hemopoietic stem cells which might
be functionally relevant to the regulation of differentiation or
at least be useful as "markers" for isolating these cells. These
arguments were in part developed in previous Wilsede symposia; here
they are further explored with particular reference to two well-characterized
cell surface glycoproteins -the gp lOO common ALL-associated antigen
and the gp 28/33 Ia-Iike or HLA-DR antigens. In addition a systemic
comparison of leukemic cells and their "presumed" equivalent normal
counterparts using a panel of monoclonal antibodies is described.
B. The Terminal Transferase Positive "Lymphocyte" in Normal Bone
Marrow
has the Same Composite Cell Surface Phenotype as Common Acute Lymphoblastic
Leukemia (cALL)
Rabbit antisera to non-T, non-B ALL have defined an antigen present
on leukemic cells from 75% of children with ALL (common ALL) and
on blast cells in some cases of AUL and CML in blast crisis (reviewed
in Greaves and Janossy 1978; Greaves 1979a). The cell surface polypeptides
(gp 100) reactive with anti-cALL have been isolated and characterized
(Sutherland et al. 1978; Newman et al. 1981; Newman et al., this
volume). Antisera with a similar if not identical specificity have
now been produced by other laboratories (Borella et al. 1977; Netzel
et al. 1978 ; Pesando et al. 1979; Kabisch et al. 1979 ; LeBien
et al. 1979), including a monoclonal antibody -1-5 (Ritz et al.
1980). Some of these sera, including the monoclonal J -5, also appear
to precipitate a cell surface glycoprotein of 95-100,000 da]tons
(Billing et al. 1978; Pesando et al. 1980) ; however, several of
these authors were unable to find normal bone marrow cells reacting
with their reagents and therefore concluded that the latter could
be identifying an antigen(s) unique to leukemic cells. We have documented
elsewhere the evidence that the cALL antigen as detected by our
particular rabbit antibodies is present on small numbers of "lymphoid"
cells in normal bone marrow and particularly in regenerating marrows
of pediatric patients (Greaves et al. 1978 ; 1980; 1anossy et al.
1979). Furthermore, agp lOO molecule can be isolated from these
sources with anti-ALL sera (Newman et a]. 1981 and this volume).
Although some one-third of cALL have a "pre-B" (µ chain positive)
phenotype (Vogler et a]. 1978; Brouet et al. 1979; Greaves et al.
1979), the majority express no markers of mature T and B cells and
presumably represent hemopoietic precursor cells in maturation arrest
( Greaves and 1 anossy 1978 ) ; whilst they are likely to be lymphoid,
i.e. precursors committed to T and/or B lineages, this is not formally
proven. These leukemias also express the nuclear enzyme TdT which
can be identified by fluorescent antibodies (Bollum 1979). A small
proportion of normal lymphoid cells in bone marrow ( as well as
most cortical thymocytes) contain TdT (Bollum 1979) ; this enzyme,
therefore, provides a very convenient single cell marker against
which cell surface phenotype can be analysed. We reported previously
that the TdT positive cell in normal bone marrow expressed the cALL
and Ia-Iike antigens but not T cell antigens or Ig (Janossy et al.
1979). We have now assessed the composite antigenic phenotype of
TdT -positive marrow cells using an extensive library of monoclonal
antibodies ( Greaves 1981 a, b) . The results (Table 1) indicate
that the majority of TdT positive cells in bone marrow have a cell
surface phenotype that is a replica of that seen in common ALL (Greaves
1981a, b; Greaves and 1anossy 1978) and which includes no exclusive
markers of either non-lymphoid lineages or mature T and B cells.
The antigenic determinant detected by monoclonal PI153/3 which is
present on most normal TdT -positive cells is, however, present
on normal B cells as well as pre-B cells and cALL (Greaves et al.
1980). It should also be noted that the majority (90% ) of TdT -positive
cells in normal bone marrow do not express any of the T lineage
antigens detected by the OKT series of monoclonals, including those
that are reactive with some or most TdT -positive T-ALL (Reinherz
et al. 1979a, 1980). An exception to this is OKT10 which, though
reactive with most T-ALL (Janossy et al. 1978a), is also present
on the majority of cALL and AML (Greaves et a].1981). Of 25 marrows
analysed (donors 2-41 years) with monoclonal (1-5) anti-ALL, 21
showed positive reactivity on 2 %-39% positive cells. This was variable
in intensity but occasionally quite bright (Fig. la). There was
a high degree of concordance with the TdT -positive cells (Table
1, Fig. 1 c) in pediatric samples as previously reported with rabbit
antisera (1anossy et al. 1979). Since monoclonal 1- 5 gives completely
concordant reactivity pattern on more than 200 leukemias assessed
(Ritz et al. 1980; M.F. Greaves and 1. Ritz, unpublished work) and
co-redistributes on the cell surface with rabbit anti-ALL (Fig.
Id), then the simplest explanation is (a) that it can recognise
the same structure (though possibly not the same determinants) as
rabbit anti-ALL and (b) that this structure, or one similar to it
[ since a family of gp lOO molecules may exist (Pesando et al. 1980)],
is present on normal TdT -positive lymphoid cells in bone marrow.
More detailed biochemistry is now required to determine the degree
of similarity between the gp lOO molecules from cALL and normal
bone marrow cells. Another monoclonal antibody reactive with cALL
has recently been described [BA-2 (Kersey et al. 1981)]. In contrast
to 1-5 and rabbit anti-cALL, this antibody appears to identify a
p 24 structure; it is also present on a small number of normal bone
marrow cells. This analysis indicates therefore that the composite
antigenic phenotype of cALL mirrors that of a normal (TdT+) cell
type in bone marrow. We presume therefore that (a) these determinants
are most likely normal gene products of hemopoietic precursors that
continue to be co-ordinately expressed in leukemia and (b) that
the cALL+ TdT+- normal cell which is restricted to bone marrow (Greaves
et al. 1979; Janossy et al. 1979) is either the major "target" population
for cALL and/or represents a post-target developmental level of
maturation arrest in ALL [ as evidenced for example by cALL blast
crises of CML (Greaves and Janossy 1978)].
Table I. Monoclonal antibody reactivity
with TdT -positive bone marrow lymphocytes and cALL a
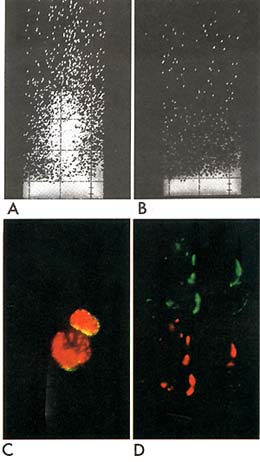
Fig. 1. Reactivity of normal and leukaemic cells
with monoclonal 1-5 anti-ALL (gp100) antigen. A,B. FACS analysis.
Vertical axis, relative fluorescence intensity; horizontal axis,
relative cell size (light scattering). Uninvolved bone marrow from
a child with rhabdomyosarcoma was stained with 1 -5 anti -ALL (
A) or control mouse ascites Ig (B). C. Normal paediatric bone marrow
cells stained (in suspension) with monoclonal 1-5 anti-cALL (gp100)
plus (after cytospin preparation and fixation) rabbit anti- TdT.
Cell surface stains green/yellow for the cALL antigen and nucleus
red/orange for TdT. D. ALL cell line (Nalm-l) cells stained first
with rabbit anti-cALL (gp 100) under capping conditions rhodamine
labelled goat anti-rabbit Ig added at 37° for 30 mins. Cells were
then kept in the cold (4°) with sodiumazide and stained with mouse
monoclonal 1-5 anti-ALL followed by fluorescein labelled goat anti-mouse
Ig. Field of 4 cells was photographed using filters for rhodamine
( upper half of picture) then moved slightly to re-expose same photograph
frame for fluorescein (lower half of picture ). Note complete co-incidence
of red and green images indicates co-redistribution of the rabbit
and mouse antibodies
C. The Cellular Selectivity of HLA-DR Expression in Leukemia
Parallels Its Presence on
Hemopoietic Progenitor Cells of the Myeloid and Erythroid Lineages
The Ia-Iike, p28,33 or HLA-DR antigens (MoIler 1976) are present
on pre-B cells, B lymphocytes, a T cell subset macrophages and different
types of epithelia, e.g. thymic, intestinal and lactating mammary.
Plasma cells, thymocytes and most T cells have no demonstrable cell
surface HLA-DR. Heteroantisera and allo-antisera to these molecules
react with B cell leukemias (e.g. CLL) as well as almost all cases
of non -TALL ( Greaves and Janossy 1978). More surprisingly, AML
(Schlossman et al. 1976; Janossy et al. 1978b) and CML in "myeloid"
blast crisis (Janossy et al. 1977) were found to express HLA-DR
or Ia-Iike antigens. These observations have now been rationalized
by reference to HLA-DR expression on normal hemopoietic precursors.
Thus some normal immature myeloblasts may express Ia-like antigens
(Ross et al. 1978 ; Winchester et al. 1977). CFU-GM activity in
vitro can be inhibited by pretreating with anti-Ia-like reagents
and complement, (Koeffler et al. 1979; Moore et al. 1980) and CFU-GM
can be positively selected on the fluorescence-activated cell sorter
(F ACS) using rabbit antibodies to the p28,33, Ia-Iike or HLA-DR
polypeptide complex (Janossy et al. 1978a). These observations have
now been confirmed and extended using a monoclonal antibody [D A2
(Brodsky et al. 1979) ] to a monomorphic or conserved determinant
of HLA-DR. Table 2 lists the leukemias that show reactivity with
this antibody. Acute myeloblastic leukemias are usually but not
invariably positive with anti-HLA-DR, whereas acute promyelocy tic
and chronic granulocytic leukemias are negative, which further emphasizes
the inverse association between HLA-DR expression and granulocytic
maturation. Notice that erythroleukemias are consistently HLA-DR
negative (Table 2). This observation is of some importance in relation
to two other reported observations: (a) that both BFU-E and CFU-E
can be inhibited by rabbit anti-p28/34 and complement (Moore et
al. 1980; Winchester et al. 1978) and (b) that rabbit anti-glycophorin
may detect "cryptic" early erythroid leukemias which would otherwise
escape this differential identification (Andersson et al. 1979,
1980 and see also Andersson, this volume). We have used both "conventional"
antisera to glycophorin and a monoclonal antibody [LICR.LON.R10
(Edwards 1980)] to screen large numbers of different leukemias.
To date we have detected three cases of glycophorin positive acute
leukemias that were not overtly erythroid.
Table 2. Reactivity of different
leukemic cells with monoclonal anti-HLA-DR (DA2)a
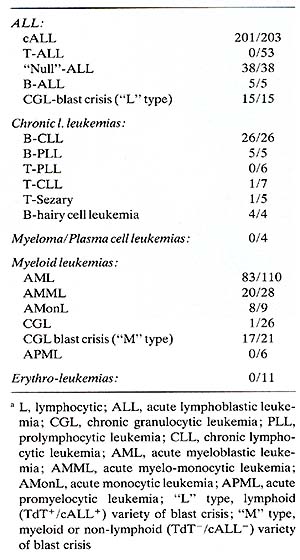
Two were CML in blast crisis and one was a child with poorly differentiated
acute leukemia (Greaves 1981 a). In these cases a proportion of cells
also reacted with monoclonal and polyclonal anti-HLA-DR; however,
double labelling showed that glycophorin and HLA-DR were present almost
exclusively on different cells. To explore further the significance
of erythroleukemic phenotypes in relation to normal early erythroid
differentiation we have labelled normal bone marrow cells with various
monoclonal antibodies, separated positive and negative cells under
sterile conditions using the FACS and assayed for BFU-E and CFU-E
activity. The details of these results are published elsewhere (Robinson
et al. 1981 ) and summarized as a 'model' diagram in Fig. 2. BFU-E
are predominantly HLA-DR+, HLA-ABC+, and glycophorin- ; CFU-E are
predominantly HLA-DR -, HLA-ABC+, and glycophorin- .All morphologically
recognisable erythroid cell precursors are HLA-DR -, HLA-ABC+or-,
and glycophorin +or-. All erythroid progenitors (BFU-E and CFU-E)
were in addition reactive with monoclonal anti-blood group A (in an
A + donor) but unreactive with OKTl, OKTll and 1-5 (see Table 1).
As an incidental observation in these experiments ( since the cultures
were all set up with erythropoietin) we noted that CFU-GM and CFU-Eo
when present also localized predominantly in the HLA-DR +, HLA-ABC+,
glycophorin- population. These observations, therefore, establish
as directly as is currently possible that HLA-DR antigens are indeed
expressed on committed hemopoietic progenitor cells [ although they
may be absent from pluripotential stem cells (Basch et al. 1977; Moore
et al. 1980) and raise the possibility that cell interactions involving
HLA-DR or Ia-like antigens might play a role in early hemopoiesis
as well as in immune responses (McDevitt 1978). Since both covert
and overt erythroleukemias are glycophorin+, HLA-DR- we can place
their likely dominant maturational arrest position close to the post-CFU
cells. However , erythroleukemia can almost certainly originate in
a pluripotential progenitor cell, since it regularly involves a granulocytic
component or may indeed occur in Ph 1 positive CML. These studies
with monoclonal antibodies confirm that glycophorin may provide a
useful marker for cryptic early erythroleukemia (Andersson et al.
1979, 1980) but also indicates that many more HLA-DR + or HLA-DR-
acute leukemias corresponding to BFU -E or CFU- E, respectively, might
exist but remain undetected as such since no exclusive marker for
these early erythroid cells yet exists.
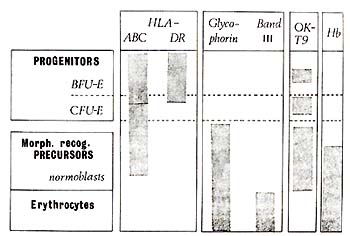
Fig. 2. Patterns of cell surface antigen expression
during erythroid differentiation. Hb, haemoglobin
D. Conclusions
Detailed serological analysis of leukemic cell surfaces using both
conventional and monoclonal antibodies indicates that acute leukemic
cells have composite antigenic phenotypes that appear to correspond
to their lineage affiliation and "position" of maturation arrest.
If leukemia specific antigens exist then they are not readily revealed
by this type of investigation. Although leukemic cells appear to
show a remarkable fidelity of phenotype, the degree to which this
is an exact replica of the normal counterpart is still open to question.
Subsequent analyses with monoclonal antibodies could identify novel
antigens perhaps restricted to individuals or small subsets of paients
or occurring in association with particular chromosomal alterations
(Rowley 1978). Karyotypic data suggest that gene dosage effects
might have a critical bearing on leukemia (see G. Klein, this volume)
and, similarly, quantitative rather than qualitative alterations
in cell surface antigens might be important. Finally, some putative
anomalies in antigenic expression are encountered in studies on
human leukemic cells (Shumak et al. 1975 ; Bradstock et al. 1980;
Greaves 1979c, 1980), although it can be ruled out that these examples
also reflect our ignorance of the heterogeneity of normal immature
cell phenotypes. Since acute leukemia is generally regarded as a
fairly high grade malignancy, it is of some interest to find that
cell surface phenotypes are conserved or only marginally altered,
suggesting an analogy with "minimally-deviated" hepatomas (Potter
1978). This permits some speculation about the contribution of the
cell surface in malignancy (Greaves 1979b) and, as shown above,
reveals characteristics of normal hemopoietic progenitors.
Acknowledgments
This work was supported by the Imperial Cancer Research Fund.
We are grateful to those colleagues listed in the references in
Table 1 who also supplied monoclonal antibodies used in part of
this study.
References
Andersson L, Gahmberg CG, Teerenhovi L, Vuopio P (1979) Glycophorin
A as a cell surface marker of early erythroid differentiation in
acute leukemia. Int J Cancer 23 :717-720
- Andersson LC, Wegelius R, Borgström GH, Gahmberg CG (1980) Change
in cellular phenotype from lymphoid to erythroid in a case of ALL.
Scand J Haematol 24:115-121
-Baldwin R (1974) Tumour specific antigens. In: The physiopathology
of cancer. Vol. 1, pp 334-392
-Basch RS, Janossy G, Greaves MF (1977) Murine pluripotential stem
cells lack la antigen. Nature, 270:520-522
-Billing R, Monowada J, Cline M, Clark B, Lee K (1978) Acute lymphocytic
leukemiaassociated cell membrane antigen. J Natl Cancer Inst 61:423-429
- Bollum F (1979) Terminal deoxynucleotidyl transferase as a hemopoietic
cell marker. Blood 54: 1203-1215
-Borella L, Sen L, Casper JT (1977) Acute lymphoblastic leukemia
(ALL) antigens detected with antisera to E rosette-forming and non-E
rosette-forming ALL blasts. J Immunol 118:309-315
- Bradstock KF, Janossy G, Bollum FJ, Milstein C ( 1980) Anomalous
gene expression in human thymic acute lymphoblastic leukaemia (ThyALL).
Nature 284:455-457
-Breard JM, Reinherz EL, Kung PC, Goldstein G, Schlossman SF ( 1979)
A monoclonal antibody reactive with human peripheral blood monocytes.
J Immunol 124: 1943-1948
-Brodsky FM, Parham P, Barnstable CJ , Crumpton MJ, Bodmer WF (
1979) Hybrid myeloma monoclonal antibodies against MHC products.
Immunol Rev 47:3-61
-Brooks DA, Beckman I, Bradley J, McNamara PJ, Thomas ME, Zola H
(1980) Human lymphocyte markers defined by antibodies derived from
somatic cell hybrids. I. A hybridoma secreting antibody against
a marker specific for human B lymphocytes. Clin Exp Immuno1 39:477-485
-Brouet JC, Preud'homme JL, Penit C, Valensi F, Rouget P, Seligmann
M (1979) Acute lymphoblastic leukemia with pre-B characteristics.
Blood 54:269-273
- Brown G, Biberfeld P, Christensson B, Mason D ( 1979) The distribution
of HLA on human lymphoid, bone marrow and blood cells. EurJ Immunol9:
272-275
- Coggin JH (1978) Retrogressive differentiation in cancer. In:
Castro J ( ed) Immunological aspects of cancer. MTP , Lancaster,
pp 89-100
-Edwards PAW (1980) Monoclonal antibodies that bind to the human
erythrocyte-membrane glycoproteins glycophorin A and Band III. Biochem
Soc Trans 8: 334-335
-Greaves MF (1979a) Immunodiagnosis of leukaemia. In: Herberman
RH, Mclntire KR (Eds ) Immunodiagnosis of cancer. Dekker, New York,
pp 542-587
- Greaves MF (1979b) Tumour markers, phenotypes and maturation arrest
in malignancy: A cell selection hypothesis. In: Boelsma E, Rümke
P (eds) Tumour Markers. Elsevier, Amsterdam, pp 201-211
-Greaves MF ( 1979c ) Cell surface characteristics of human leukaemic
cells. In: Campbell PN , Marshall RD (eds) Essays in biochemistry.
Academic Press, New York, pp 78-124
-Greaves MF (1980) Analysis of human leukaemic cell populations
using monoclonal antibodies and the fluorescence activated cell
sorter. In: Yohn DS, Lapin BA, Blakeslee JR ( eds ) Advances in
comparative leukemia research 1979. Elsevier/North Holland, Amsterdam,
New York, pp 235-242
-Greaves MF ( 1981 b) Analysis of lymphoid phenotypes in acute leukaemia:
Their clinical and biological significance. Cancer Res, in press
-Greaves MF, J anossy G (1978) Patterns of gene expression and the
cellular origins of human leukaemias. Biochim Biophys Acta 516:
193-230
- Greaves MF, Delia D , Janossy G, Rapson N, Chessells J, Woods
M, Prentice G (1980) Acute lymphoblastic leukaemia associated antigen.
VI. Expression on non-leuka ellic 'lymphoid' cells. Leuk Res 4:
15-32
- Greaves MF, Janossy G, Francis GE, Minowada J (1978) Membrane
phenotypes of human leukemic cells and leukemic cell lines. Clinical
correlates and biological implications. In: Differentiation of normal
and neoplastic hematopoietic cells. Cold Spring Harbor Laboratory,
New York, pp 823-841
- Greaves MF, Verbi W, Vogler L, Cooper M, Ellis R, Ganeshaguru
K, Hoffbrand V, J anossy G, Bollull FJ: Antigenic and enzymatic
phenotypes of the pre-B subclass of acute lymphoblastic leukaemia.
Leuk Res 3:353-362
- Greaves MF, Verbi W, Kellshead J, Kennett R (1980) A monoclonal
antibody identifying a cell surface antigen shared by common acute
lymphoblastic leukemias and B lineage cells. Blood 56: 1141-1144
- Hakollori S (1975) Structure and organization of cell surface
glycolipids : dependency on cell growth and malignant transformation.
Biochill Biophys Acta417 : 55-89
-Janossy G, Greaves MF, Sutherland R, Durrant J, Lewis C (1977)
Comparative analysis of membrane phenotypes in acute lymphoid leukaemia
and in lymphoid blast crisis of chronic myeloid leukaemia. Leuk
Res 1 :289-300
- Janossy G, Francis GE, Capellaro D, Goldstone AH, Greaves MF (1978a)
Cell sorter analysis of leukaellia-associated antigens on human
myeloid precursors. Nature 276: 176-178
- Janossy G, Goldstone AH, Capellaro D, Greaves MF, Kulenkallpff
J, Pippard M, Welsh K (1978b) Differentiation linked expression
of p28,33 (Ia-like ) structures on human leukaemic cells. Br J Haematol
37:391-402
-Janossy G, Bollull F, Bradstock K, Rapson N, Greaves MF (1979)
Terminal deoxynucleotidyl transferase positive human bone marrow
cells exhibit the antigenic phenotype of common acute lymphoblastic
leukaemia. J Illmunol 123:1525-1529
-Kabisch H, Arndt R, Becker W-M, Thiele H-G, Landbeck G (1979) Serological
detection and partial characterization of the common-ALL-cell associated
antigen in the serum of cALL-patients. Leuk Res 3:83-91
- Kennett RH, Gilbert F (1979) Hybrid myelomas producing antibodies
against a human neuroblastoma antigen present on fetal brain. Science
203: 1120-1121
-Kersey JH, LeBien TW, Abrallson CS, Newman R, Sutherland R, Greaves
M ( to be published) gp24 : a human hemopoietic progenitor and acute
lymphoblastic leukellia-associated cell surface structure identified
with monoclonal antibody. J Exp Med 153:726-731
- Koeffler HP, Niskanen E, Cline M, Billing R, Golde D (1979) Human
myeloid precursors forming colonies in diffusion chambers expresses
the Ia-Iike antigen. Blood 54: 1188-1191
- Kung PC, Goldstein G, Reinherz EL, Schlossman SF (1979) Monoclonal
antibodies defining destinctive human T cell surface antigens. Science
206: 347-349
-LeBien TW, Hurwitz RL, Kersey JH (1979) Characterization of a xenoantiserull
produced against three molar KC1-solubilized antigens obtained from
a non- T, non-B (pre-B) acute lymphoblastic leukemia cell line.
J Illmunol122 : 82-88
- Levy R (to be published) In: Cell markers and acute leukaemia.
Cancer Treat Rep -Marchesi VT (ed) (1976) Membranes and neoplasia.
Prog Clin BioI Res 9
-McDevitt HO (1978) la antigens and Ir genes. Academic Press, London
New York -McMichael AJ, Pilch JR, Galfre G, Mason DY, Fabre JW,
Milstein C (1979) A human thymocyte antigen defined by a hybrid
myeloma monoclonal antibody. Eur J Illmunol 9:205-210
- Moller G (ed) (1976) Transplant Rev 30 -Moore MAS, Broxmeyer HE,
Sheridan APC, Meyers PA, J acobsen N, Winchester RC (1980) Continuous
human bone marrow culture: la antigen characterization of probable
pluripotential stem cells. Blood 55 :682-690
-Netzel B, Rodt H, Lau B, Thiel E, Haas RJ, Dormer P, Thierfelder
S (1978) Transplantation of syngeneic bone marrow incubated with
leukocyte antibodies. II. Cytotoxic activity of anti-cALL globulin
on leukemic cells and normal hemopoietic precursor cells in Man.
Transplantation 26:157-161
- Newman RA, Sutherland R, Greaves MF (1981) The biochemical characterization
of a cell surface antigen associated with acute lymphoblastic leukemia
and lymphocyte precursors J Illmunol, in press
-Pesando JM, Ritz J, Lazarus H, Baseman Costello S, Sallan S, Schlossman
SF (1979) leukellia-associated antigens in ALL. Blood 54: 1240-1248
- Pesando JM, Ritz J, Levine H, Terhorst C, Lazarus H, Schlossman
SF (1980) Human leukellia-associated antigen: Relation to a family
of surface glycoproteins. J Illmunol 124:2794-2799
-Potter VR ( 1978) Phenotypic diversity in experimental hepatomas:
the concept of partially blocked ontogeny. Br J Cancer 38: 1-23
-Reinherz EL, Kung PC, Goldstein G, Schlossman SF (1979a) Separation
of functional subsets of human T cells by a monoclonal antibody.
Proc Natl Acad Sci USA 76:4061-4065
-Reinherz EL, Kung PC, Goldstein G, Schlossman SF (1979b) A monoclonal
antibody with selective reactivity with functionally mature human
thyllocytes and all peripheral human T cells. J Illmunol 123: 1312-1317
-Reinherz EL, Kung PC, Goldstein G, Levey RH, Schlossman SF (1980)
Discrete stages of human intrathymic differentiation: Analysis of
normal thyllocytes and leukemic lymphoblasts of T lineage. Proc
Natl Acad Sci USA 77: 1588-1592
- Ritz J, Pesando JM, Notis-McConarty J, Lazarus H, Schlossman SF
(1980) A monoclonal antibody to human acute lymphoblastic leukaemia
antigen. Nature 283: 583-585
-Robinson J, Sieff C, Delia D, Edwards P, Greaves M (1981) Expression
of cell surface HLA-DR, HLA-ABC and glycophorin during erythroid
differentiation. Nature 289:68-71
-Ross GD, Jarowski GI, Rabellino EM, Winchester RJ (1978) The sequential
appearance of Ia-like antigens and two different complement receptors
during the maturation of human neutrophils. J Exp Med 147:730-744
-Rowley JD (1978) Chromosomes in leukaemia and lymphoma. Semin Haematol
15:301-319
-Schlossman SF, Chess L, Humphreys RE, Strom inger JL (1976) Distribution
of la-like molecules on the surface of normal and leukemic human
cells. Proc Natl Acad Sci USA 73: 1288-1292
- Shumak KH, Rachkewich RA, Greaves MF (1975) I and i antigens on
normal human T and E lymphocytes and on lymphocytes from patients
with chronic lymphocytic leukaemia. Clin Immunol Immunopathol 4:
241-247
-Sutherland R, Smart J, Niaudet P , Greaves MF (1978) Acute lymphoblastic
leukaemia associated antigen. II. Isolation and partial characterisation.
Leuk Res 2:115-126
- Vogler LE, Crist WM, Eockman DE, Pearl ER, Lawton AR, Cooper MD
(1978) Pre-E cell leukemia. N Engl J Med 298: 872-878
- Wallach DFH ( ed) ( 1978) Membrane anomalies of tumor cells. Karger,
Easel -Winchester RJ, Ross GD, Jarowski CI, Wang CY, Halper J, Eroxmeyer
HE ( 1977) Expression of la-Iike antigen molecules on human granulocytes
during early phases of differentiation. Proc Natl Acad Sci USA 74:4012-4016
-Winchester RJ, Meyers PA, Eroxmeyer HE, Wang CY, Moore MAS, Kunkel
HG (1978) Inhibition of human erythropoietic colony formation in
culture by treatment with la antisera. J Exp Med 148: 613-618
|



