| Purification
to Apparent Homogeneity and Biochemical Characterization of Human Pluripotent Hematopoietic ColonyStimulating Factor * |
|
* Laboratory of Developmental Hematopoiesis and
Laboratory of Molecular Hematology, Memorial Sloan-Kettering Cancer
Center, New York, NY 10021
Colony-stimulating factors (CSFs) are a family of hematopoietic growth factors required for the proliferation and differentiation of hematopoietic progenitor cells [I, 2]. In the human system, purification to homogeneity and biochemical characterization has only been reported for macrophage-active CSF (CSF-I) [3]. However, there are many reports about highly purified human granulocyte-macrophage CSFs ( e.g. [4- 7]), but not about pluripotent human CSF. Assays are available to detect human clonogenic precursors that give rise to cells of the erythroid, granulocytic, megakaryocytic, macrophage, colony-forming unit granulocytes, erythrocytes, macrophages, and megakaryocytes, CFU-GEMM, and possibly lymphoid lineages [8-10]. CSFs with activities on these pluripotential progenitor cells (pluripotent CSF) are produced by mitogen- or antigen-activated T -lymphocytes [11] and by human tumorcell lines [12] or HTLV-transformed lymphoid cells [ 13]. We report in this paper the purification to homogeneity and biochemical characterization of a human pluripotent CSF, produced and released by the human bladder carcinoma-cell line 5637.
Gran ulocyte- macrophage-CSF ( G M -CSF), gran ulocyte- macrophage-eryth rocyte-me ga karyocyte-CSF (GEMM-CSF), and early erythroid burst-forming unit (BFU-E) activities were tested on low-density, T -celldepleted, nonadherent human bone marrow cells as described [14-15] and detailed in another paper by Platzer et al. in this volume. For assay of differentiation induction, the method of Metcalf [ 16] was used, whereby pluripotent CSF was added to cultures of the murine myelomonocytic WEHI-3B(D + ) or the human promyelocy tic HL-60 leukemic cells and scored for differentiation on day 7 and 14 respective ly. As shown in the Results, a single protein stimulates colony formation by CFUGEMM, BFU-E, and CFU-GM progenitor cells. We termed this protein "pluripotent CSF" or "pluripoietin ". Due to the low numbers of mixed colonies per dish attain able in this assay system, titration of test samples for determination of pluripotent CSF activity presented difficulties in quantitation. Therefore, we used the GM-CSF assay as described [14,15] to measure the GM-CSF aspect of the pluripotent CSF in the samples that supported growth of CFUGEMM and BFU-E for calculating the specific activities throughout the purification procedure. Table 1. Purification of human pluripotent
CSF 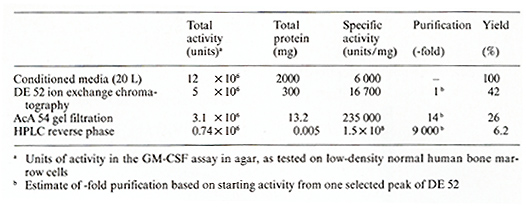
The human bladder carcinoma-cell line 5637 has been reported to produce constitutively a GM-CSF [17] and GEMMCSF [12]. The cells were cultured in RPMI 1640 supplemented with glutamine (2 mM), antibiotics, and 10% fetal calf serum (FCS). For production of pluripotent CSF used for purification cells were kept for 48- 72 h in medium containing low serum (0.2% FCS). The conditioned medium from low-serum-containing cultures was harvested and used for purification. The first three steps of purification involved ammonium sulfate precipitation (80% saturation), anion-exchange chromatography [ diethylaminoethanol (D EAE)-cellulose, DE 52, Whatman, Clifton, NJ], and gel filtration (AcA 54 Ultrogel, LKB Products, Inc., Rockland, MD) (Table I). These steps were used because they were highly effective for other cytokines, notably Interleukin 2 [18] and B-cel1-differentiating factor [19] and have been described in detail elsewhere [ 18]. Pluripotent CSF eluted from the DE 52 cellulose column between 0.075 and 0.1 M NaCl in 0.05 M Tris/HCl, pH 7.8, and from the AcA 54 column with a single peak at around 32000 molecular weight. The final step involved chromatography on a reverse-phase high-perforfiance liquid chromatography (HPLC) column (uBondapak C 18, Waters) and a Waters HPLC system using l-propanol as organic solvent (20%-50% I-propanol gradient in 2 h) and a buffer system con taining 0.9 M acetic acid and 0.2 M pyridine, pH 4.0. Pluripotent CSF activity eluted as a single peak at 42% I-propanol. The purification schedule with degree of purification of pluripotent CSF as measured by GM-CSF activity, protein content, specific activity, and yield is detailed in Table I. We obtained a specific activity of 1.5 X 10 high 8 U/ mg protein.
The final preparation obtained after HPLC was analyzed on a 15%
sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-P
AGE) gel [20] followed by the sensitive silver staining technique
(Biorad Lab., Rockville Centre, NY; Fig. 1 ). Only one protein band
with a molecular weight of 18000 was seen under reducing (5% 2-mercaptoethanol;
Fig. 1) and non reducing (not shown) conditions. After electrophoresis
under nonreducing conditions, a parallel gel was sliced into 2-mm
sections and proteins eluted from each slice into phosphate buffer.
Pluripotent CSF was found to be localized in the slice number corresponding
to 18000 molecular weight (Fig. I). Reelectrophoresis of the protein
in the active slice-fraction with SDS-P AGE under reducing conditions
revealed again a protein band of 18000 molecular weight (not shown
). The purified pluripotent CSF was also subjected to isoelectrofocusing
analysis in an IEF column (LKB 8100) [ 18] using ampholines with
a pH range of 3.5-10. Pluripotent CSF was localized in one fraction
with a pH of 5.5 (Table 2). 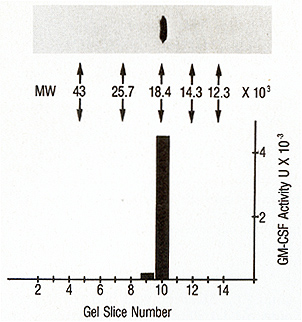

Fifty units of GM-CSF activity of pluripotent CSF (1.8 X 10 high -11 M) supported the half-maximal cloning of CFU-GM, while 500 U /ml was needed to support the cloning of human CFU-GEMM and BFU-E. In addition pluripotent CSF at a concentration of between 500 and 1000 U /ml was capable of inducing differentiation of the leukemic cell lines HL-60 and WEHI 3B (0 + ). A detailed biological characterization of pluripotent CSF is described in the paper by Platzer et al. in this volume.
The protein described in this paper is capable of stimulating the in vitro growth of human mixed colony progenitor cells (CFU-GEMM), early erythroid progenitor cells (BFU-E), and granulocyte-macrophage progenitors (CFU-GM) and in addition induces differentiation of the murine myelomonocytic (WEHI-3B (0 + )) and the human promyelocytic (HL-60) leukemic cell lines. It has a molecular weight of 18 000 and an isoelectric poin t of 5.5. The specific activity is 1.5xl0 high 8U/mg protein. The purified protein, shown in Fig. I, and the pluripotent CSF activity are identical because: (I) protein and activity eluted in the same fraction from the HPLC; (2) we were not able to separate biological activity and the 18 000 molecular weight protein by using additional HPLC columns (Oiphenyl, C4, Hydroxylapatite) and buffer systems; (3) identical localization of protein and activity in SDS-PAGE (Fig. I); (4) high specific activity (1.5xI0 high 8U/mg protein; 1 U=3.7x 10high-13 M), which is comparable to pure murine CSF [21] and human CSF-l [3]. Therefore, it is very unlikely that pluripotent CSF activity is not associated with the 18 000 molecular weight protein. The availability of purified human pluripotent CSF has important and farreaching implications for the analysis of human hematopoiesis and possibly for the understanding and management of clinical diseases involving hematopoietic derangement or failure.
We would like to thank Ms. Maureen Sullivan and Mr. John Foster for excellent technical assistance.
1. Burgess A W, Metcalf O ( 1980) The nature and action of granulocyte-macrophage colony-stimulating factors. Blood 56: 947 -958 2. Nicola NA, Vadas M (1984) Hematopoietic colony-stimulating factors. Immunol Today 5:76-81 3. Das SK, Stanley ER (1982) Structure-function studies of a colony stimulating factor (CSF-l). J BioI Chell 257: 13679-13 684 4. Wu MC, Yunis AA (1980) Common pattern of two distinct types of colony-stimulating factor in human tissues and cultured cells. J Clin Invest 65: 772- 775 5. Lusis AJ, Quon OH, Golde OW (1981) Purification and characterization of a human T -lymphocyte-derived granulocyte-llacrophage colony-stillulating factor. Blood 57:13-21 6. Abboud CN, Brennan JK, Barlow GH, Lichtman MA (1981) Hydrophobic adsorption chromatography of colony-stimulating activities and erythroid-enhancing activity from the human monocyte-like cell line CGT. Blood 58: 1148-1154 7. Motoyoshi K, Suda T, Kusulloto K, Takaku F, Miura Y (1982) Granulocyte-llacrophage colony-stimulating and binding activities of purified human urinary colony-stimulating factor to murine and human bone marrow cells. Blood 60: 1378-1391 8. Fauser AA, Messner HA (1978) Granulocyte-erythropoietic colonies in human bone marrow, peripheral blood and cord blood. Blood 52: 1243-1248 9. Fauser AA, Messner HA (1979) Identification of megakaryocytes, macrophages and eosinophils in colonies of human bone marrow containing neutrophilic granulocytes and erythroblasts. Blood 53: 1023-1027 10. Messner HA, Izaguirre CA, Jallal N (1981) Identification of T lymphocytes in human mixed hematopoietic colonies. Blood 58: 402-405 11. Ruppert S, Lohr GW, Fauser AA (1983) Characterization of stimulatory activity for human pluripotent stem cells (CFUGEMM). Exp Hematolll: 154-161 12. Myers CO, Katz FE, Joshi G, Millar JL ( 1984) A cell line secreting stimulating factors for CFU-GEMM culture. Blood 64: 152-155 13. Salahuddin SZ, Markhall PO, Lindner SG, Gootenberg J, Popovic M, Hellmi H, Sarin PS, Gallo RC (1984) Lymphokine production by cultured human T cells transformed by human T cell leukellia-lymphoma virus-l. Science 223:703-707 14. Broxmeyer HE, Moore MAS, Ralph P (1977) Cell-free granulocyte colony inhibitory activity derived from human polymorphonuclear neutrophils. Exp Hematol 5: 87-102 15. Li L, Broxmeyer HE, Meyers PA, Moore MAS, Tzvi Thaler H (1983) Association of cell cycle expression of Ia-like antigenic determinants on normal human multipotential (CFU-GEMM) and erythroid (BFU-E) progenitor cells with regulation in vitro by acidic isoferritins. Blood 61: 250-256 16. Metcalf D ( 1980) Clonal extinction of myelomonocytic leukemic cells by serum from mice injected with endotoxin. Int J Cancer 25:225-233 17. Svet-Modalsky GJ, Zinzer SN, Svet-Modalsky IA, Mann PE, Holland JF, Fogh J, Arlin Z, Clarkson BO ( 1980) CSF-producing human tumor cell lines. Lack of CSF activity of human stromal bone marrow fibroblasts. Exp Hematol8 [Suppl] 7: 76 (abstract) 18. Welte K, Wang CY, Mertelsmann R, Venuta S, Feldman S, Moore MAS (1982) Purification of human interleukin 2 to apparent homogeneity and its molecular heterogenei ty .J Exp Med 165: 454-464 19. Ralph P, Welte K, Levi E, Nakoinz I, Litkovsky PB, Mertelsmann R, Moore MAS (1984) Human B cell-inducing factor(s) for production of IgM, IgG and IgA: independence from ILl. J lmmunol 132: 1858 -1862 20. Laellmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680-685 21. I hIe JN, Keller J, Henderson L, Klein F , Palaszynski E (1982) Procedures for the purification of interleukin 3 to homogeneity. J IllmunoI129:2431-2436 |