|
Hematological Scientific Center, Moscow, U.S.S.R., 125167 A. Introduction The hierarchy of hematopoietic stem cells (HSC) is represented by several categories of maturating pluripotent progenitors. Most of them are the members of transitional cell populations and, obviously, have no capacity for self-maintenance, i. e., are not capable of giving rise to self-replicating offspring with the same proliferative potential as the parent had [1]. The foundator of this hierarchy has not yet been identified. The most probable candidate at present is the cells supporting long-term hematopoiesis in vivo after repopulation of lethally irradiated or genetically defective W -mutant mice or in vitro in long-term culture. However, the self-renewal is also not proven for these cells, and hematopoiesis, at least in culture, occurs by clonal succession [2]. The experimental data support the hypothesis that even primitive HSC (PHSC) exhibit high, though limited, proliferative potential. This cell category is usually identified by competitive repopulation assay using a mixture of tested and standard cells identifiable by biochemical, immunological, karyological, or other markers [3]. Limiting dilution analysis based on the ability of small numbers of + / + hematopoietic cells to cure anemia of W -mutant mice has been also used for the determination of PHSC [4-6]. In the present study, the characteristics of PHSC were investigated in long-term bone marrow culture by both competitive repopulation and limiting dilution methods. Primitive HSC were defined as precursors responsible for the long-term maintenance of hematopoiesis, i. e., for the generation of mature as well as progenitor hematopoietic cells, particularly CFUs.
I. Mice
1. Limiting Dilution Analysis 2. Competitive Repopulation
Irradiated recipients were injected with 0.35 x 10 high 6 or 35
x 10 high 6 syngeneic bone marrow cells. PHSC determination was
performed by limiting dilution analysis 6 or 7.5 months after reconstitution.
VI. Cytostatics Hydroxyurea (900 mg/kg) was injected i. p. six times at 6-h intervals, and donor mice were killed 2 h after the last injection. 5-Fluorouracil (150 mg/kg) was injected i. v., and donor mice were killed 2 or 4 days later . A significant correlation between the number of explanted bone marrow cells and the estimated amount of PHSC was observed, the linear regression line going through the origin (Fig. 1 ). Thus, the data suggest that the number of PHSC may be determined by the limiting dilution method. The content of PHSC in murine bone marrow as estimated by this method was 90 I 20 per femur. The treatment of donor mice with cytostatics revealed that PHSC are insensitive to the drugs used. Neither intensive treatment with hydroxyurea nor the injection of 5-fluorouracil essentially reduced the number of PHSC in bone marrow in spite of the fact that both drugs killed more than 99% of CFUs (Table 1). Six or 7.5 months after the reconstitution, the number of PHSC in mice injected with a "small" dose (0.35 x 10 high 6) of bone marrow cells was lower than in Table I. The effect of phase-specific
cytostatics on CFUs and PHSC content in murine bone marrow 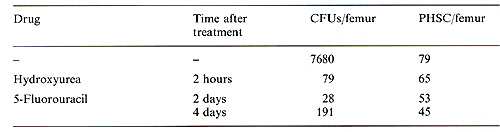 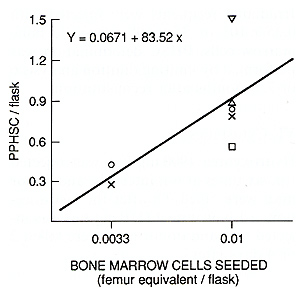 Fig. I. Primitive hematopoietic stem cell content in normal mice as measured by limiting dilution analysis 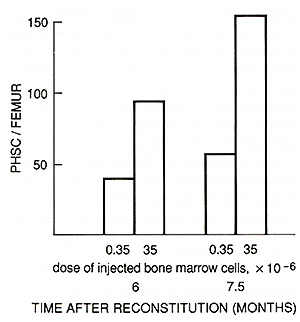 Fig. 2. Primitive hematopoietic stem cell content in mice reconstituted with different doses of bone marrow cells 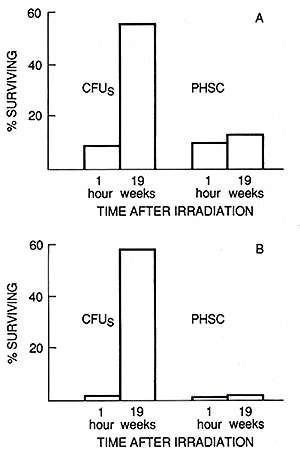
recipients seeded with a " large " one (35 x 10 high 6 ) ( Fig.2 ), altough the bone marrow cellularity and CFU content were aproximately normal and were the same in both groups of mice ( data not shown ). The content of PHSC in sublethally irradiated mice was estimated by competive repopulation 19 weeks after 2 Gyirradiation in two experiments, and 10 or 19 weeks after 4ay irradiation in five experiments. The results obtained in a representative experiment are shown in Fig. 3. Immediately after irradiation with 2 ay the number of CFUs was reduced ten times, and after 4 ay, 100 times as compared with normal bone marrow. Two months later, the CFU population was restored to a subnormallevel in both groups of mice. The competitive repopulation of cells irradiated with the same doses but harvested from mice immediately after irradiation or 10 -19 weeks later revealed equal content of PHSC. Thus, the cells responsible for the maintenance of long-term hematopoiesis in culture do not possess the capacity for regeneration after irradiation. D. Discussion This paper presents the first estimation of PHSC in murine bone marrow as measured by limiting dilution analysis of long-term cultures. The frequency of PHSC is approximately 10 per 10 high 6 bone marrow cells. This number is in good agreement with data obtained in vivo by the limiting dilution method of reconstituted W-mutant mice [4-6]. The CFU population in bone marrow of studied genotypes reached 5000- 8000 per femur . Taking the seeding efficiency factor as being 0.05-0.1 [10], the CFU population would be 50000-150000 per femur. Therefore, each pluripotent HSC is capable of producing a clone which includes 500-1500 CFUs and 1-4 x 10 high 9 differentiating cells (it should be borne in mind that an 8-day spleen colony may consist of 4 x 10 high 6 cells). Such enormous hematopoietic clones can obviously easily support the continuous production of hematopoietic cells during the whole life span of a mouse by the sequential expansion of a relatively small number of PHSC formed during embryogenesis. The hematopoietic tissue of an adult mouse is presented by approximately 4 x 10 high 8 cells. If the replacement of all hematopoietic cells in the bone marrow occurs in only 4 days, one PHSC capable of producing a clone of 10 high 9 cells would be enough for the maintenance of prolonged hematopoiesis for at least 10 days. Thus, only several hundred PHSC would be expended during the whole life span of a mouse. Therefore, although not yet conclusive, the results obtained support the hypothesis of hematopoiesis by clonal succession [ 11, 12] . The other important result obtained in this study suggests that PHSC are insensitive to cytostatics, in particular to phase-specific agents such as hydroxyurea which influence only cells synthesizing DNA. When the population of more mature precursors (CFUs) was nearly completely eliminated by cytostatics, the number of PHSC in bone marrow was unchanged. Therefore, PHSC apparently are in the a o phase of the cell cycle, and are members of a "hidden" reserve stem cell compartment. Their triggering into a state of proliferation could not be induced even by the strong hematopoietic stress. The data are in complete agreement with those obtained by competitive repopulation [3] and are not contradictory to the hypothesis that the population of "quiescent" primitive progenitors is formed during embryogenesis and is sequentially expended throughout postnatal development, producing one after another hematopoietic cell clones. If the latter were the case, then PHSC should not possess regeneration capacity. In the study performed, the increase of PHSC number until 19 weeks after 4 ay irradiation was not really detected, although almost complete reconstitution of the CFU pool took place. The higher PHSC content in mice injected with a greater dose of bone marrow cells even several months after irradiation is also in accordance with this hypothesis because, if PHSC self -maintenance ability actually exists, the number of these cells would not depend on the injected cell dose. On the whole, the data discussed above suggest that at present the hypothesis of hematopoiesis by clonal succession seems to be the most simple and requires the minimum number of additional assumptions. For the reliable discrimination between this hypothesis and the idea of the existence of immortal HSC, new data are necessary. Retrovirus-mediated gene transfer may be a very suitable system for the exploration of this intriguing problem.
1. Chertkov J L ( 1986) Early hemopoietic and stromal precursor cells. Int Rev Cytol 102:271-313 2. Chertkov JL, Deryugina El, Drize NJ, Udalov GA (1987) Individual clones of hemopoietic cells in murine long-term bone marrow culture. Leukemia 1: 491496 3. Chertkov JL, Drize NJ, Gurevitch OA, Udalov GA (1986) Cells responsible for restoration of haemopoiesis in long-term murine bone marrow culture. Leuk Res 10: 659-663 4. Boggs DR, Boggs SS, Saxe DF et al. (1982) Hematopoietic stem cells with high proliferative potential. Assay of the concentration in marrow by the frequency and duration of cure ofW /WV mice. J Clin Invest 70: 242-253 5. Mintz B, Covarrabias L, Hawley RG (1987) Hematopoietic stem cells as po tential vehicles for recombinant genes in prenatal mice. Haematologica 72 [Suppl]: 89-94 6. Nakano T, Naki N, Asai H, Kitamura y (1987) Long-term monoclonal reconstitution of crythropoiesis in genetically ane mic W /WV mice by injection of 5-fluorouracil-treated bone marrow cells of Pgk-1 b / Pgk-1a mice. Blood 70: 17581763 7. Till JE, McCulloch EA (1961) A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Rad Res 14: 213-222 8. Dexter TM, Allen TD, Lajtha LT (1977) Conditions controlling the proliferation of hemopoietic stem cells in vitro. J Cell Physiol 91: 335-344 9. Chertkov JL, Drize NJ, Gurcvitch OA, Udalov GA (1983) Hemopoietic stromal precursors in long-term culture of bone marrow. I. Precursor characteristics, kinetics in culture, and dependence on quality of donor hemopoietic cells in chimeras. Exp Hemato111:231-242 10. Siminovitch L, McCulloch EA, Till JE (1963) The distribution of colony-forming cells among spleen colonies. J Cell Comp Physiol 63: 327 -336 11. Kay HEM (1965) How many cell genera tions? Lancet I I: 418 -419 12. Micklem HS, Lennon JE, Ansell JD, Gray RA (1987) Numbers and dispersion of repopulating hematopoietic cell clones in radiation chimeras as function of injected cell dose. Exp Hematol 15: 251-257 |