In: Zander AR et al. (eds) Gene Technolgy, Stem Cell and Leukemia Research, Nato ASI Series H: Cell Biology, Vol 94, Springer-Verlag, Berlin Heidelberg New York London, pp 3-10 |
|
ICAPB University of Edinburgh West Mains Road Edinburgh
EH9 3JT Scotland lCRC Beatson Laboratories, Introduction The hematopoietic system can be considered as a spectrum of differentiation and self renewal with at one extreme terminally differentiated cells such as erythrocytes or macrophages, and at the other, pluripotential hematopoietic stem cells (HSC) , which can give rise to all the different lineages of the hematopoietic system, and due to their capacity for self renewal, persist throughout adult life (Spangrude et al. 1991 ). Introduction of limiting numbers of these cells into recipients whose own hematopoietic systems have been ablated (by, for example, irradiation) results in long-term reconstitution by the donor cells. These properties of HSC have make them tempting targets for gene manipulation, both for investigating the molecular and cellular biology of genes that are expressed in different lineages of the hematopoietic system and in somatic gene therapy. In the former case, gene manipulation of HSC would have particular benefit where the mutations, if transmitted through the germ line, would result in embryonic lethality. Gene manipulation in HSC has proved difficult, at least in part due to an inadequate understanding of the biology of these cells; addition of genes (transgenesis) is dependent on retroviral infection (Fairbairn and Spooncer 1993). Gene targeting by homologous recombination in HSC has not yet been shown to be practical. In contrast, there is considerable experience in specific and facile manipulation of the mouse genome using embryonal stem (ES) cells, which as totipotential cells, can give rise to all the different cellular lineages in the organism (Evans and Kaufman 1981, Robertson 1986) .In particular, ES cells have proved to be amenable to gene targeting by homologous recombination. Early work demonstrated the appearance of erythrocytes following differentiation of ES cell under specific conditions in vitro (Doetschman et al. 1985). Under conventional circumstances, these cells could only have arisen from differentiation of pluripotent hematopoietic precursors, themselves derived from the ES cells. ES cells are competent to produce HSC; using HSC isolated from fetal livers of mice derived in their entirety from ES cells are capable of rescuing and reconstituting secondary recipient mice (Forrester et al. 1991 ). The question of whether HSC can be derived from ES cell differentiation in vitro is currently an active area of research (Hole et al. 1994, Muller and Dzierzak 1993). However, most mature hematopoietic lineages can be detected following in vitro differentiation of ES cells (Chen et al. 1993, GutierrezRamos and Palacios 1992, Keller et al. 1993, Nakano et al. 1994, Wiles 1993). As part of our study of the hematopoietic potential of ES cells, we have examined the lymphoid commitment of ES cells differentiated in vitro. The ability to isolate T lymphocytes from ES cells in vitro may offer an alternative strategy in the study of thymocyte development and positive and negative T cell selection.
ES cell differentiation. 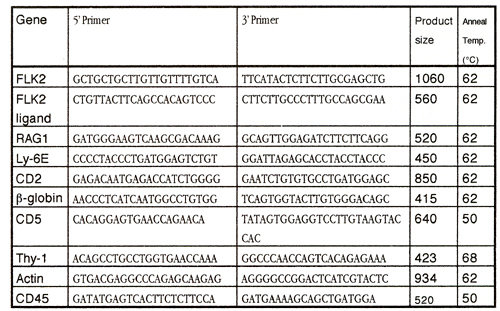
Flow cytometry The expression of surface antigens by single cells
liberated from the differentiating embryoid bodies is shown in figure
1 a-c. HSC from mice are believed to have a characteristic phenotype,
namely Ly-6E+ve, Thy-1 +ye (Spangrude, et al. 1991 ). Expression
of these antigens was assessed individually (fig.1 a and b) and
together (data not shown). Thy-1 was found on undifferentiated ES
cells, the numbers of cells expressing the antigen decreasing over
the first 5 days of differentiation and apparently increasing thereafter
to approximately 30% of embryoid body cells over the time course
studied. The numbers of cells expressing both antigens were less
than 0.1% of total cells at any time point examined. Thy-1 and Ly6E
are not HSCrestricted, and are expressed on other cell types, including
T cells. In order to examine the total hematopoietic commitment
and T -cell commitment, expression of CD45, CD3 and alfa▀TCR were
examined (Figs 1 band 1 c). CD45+ve hematopoietic cells increase
in number over the time course, from about 1% of cells at day 7
to approximately 7% of cells after 20 days of differentiation. However
, a relatively small number of these cells are likely to be T cells;
CD3 or af3 TCR +ve cells are first detected around day 10, again
increasing over the time course to about 10% of the numbers of CD45+ve
hematopoietic population. The small numbers of TCA +ve cells recovered
precluded two-colour flow cytometry. However, CD4+ve and CD2+ve
cells were also found around the time point when these T cell markers
could be detected.  These data suggested that in common with several other studies, we were able to derive lymphoid cells from ES cells by in vitro differentiation (in a separate study, B cell development was indicated by detection of IgM+ve, IgD+ve and/or B220+ve cells in differentiation at around 12 days (data not shown). To confirm these results and examine the transcription of developmentally regulated genes, we carried out a selected series of AT -PCA reactions. RT -PCR A schematic of the results obtained is shown in table 1. The integrity of the cDNA for PCR was confirmed by a positive result for actin transcripts in all of the samples. In brief, the results support the observation that Iymphoblastoid cells are appearing later in the differentiation pathway. RAG-1 expression was detected as early as day 6 after initiation of differentiation, coincident with detection of CD2 transcripts. CD45 was detected by day 8. Thy-1 and Ly-6E transcripts were detected early in differentiation; in ES cells or day 2 differentiated cells respectively. Of interest was the relatively early appearance of a marker of another hematopoietic lineage, ▀globin. However, no red blood cells were observed in embryoid bodies during this series of experiments. Detection of FLK2 ligand transcripts in differentiating ES cells was in contrast to its receptor, whose transcripts were not detected at the level of analysis adopted. 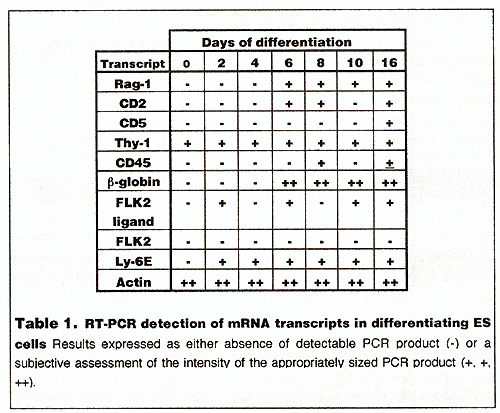
This work was supported by grants from The Wellcome Trust,
Chen, J., Gorman, J. R., Stewart, V., Williams, B., Jacks, T. and Alt, F. W. (1993) Generation of normal lymphocyte populations by Rb-deficient embryonic stem cells Curr. Bioi. 3: 405-413 Doetschman, T., Eisetter, H., Katz, M., Schmidt, W. and Kemler, R. (1985) The in vitro development of blastocyst-derived embryonic stem cell lines: formation of visceral yolk sac, blood islands and myocardium. J.Embryol.exp.morphol. 87: 27-45 Evans, M. J. and Kaufman, M. (1981) Establishment in culture of pluripotential cells from mouse embryos Nature 292: 154-156 Fairbairn, L. J. and Spooncer, E. (1993) Retroviral gene transfer into haemopoietic cells In: Haemopoiesis N. G. Testa and I. Molyneux (eds.) Oxford University Press Oxford Forrester, L. M., Bernstein, A., Rossant, J. and Nagy, A. (1991) Long-term reconstitution of the mouse hematopoietic system by embryonic stem cell derived fetalliver Proc.Natl.Acad.Sci.(USA) 89: 7514-7517 Gutierrez-Ramos, J. C. and Palacios, R. (1992) In vitro differentiation of embryonic stem cells into lymphocyte precursors able to generate T and B lymphocytes in vivo Proc.Natl.Acad.Sci.(USA) 89: 9171-9175 Hole, N., Graham, G. and Ansell, J. D. (1994) Self renewing and repopulating hematopoietic progenitors can be derived from embryonal stem cells in vitro J.Cell.Biochem. 188: 181 Hole, N. and Smith, A. G. (1994) Embryonic stem cells and hematopoiesis In: Culture of hematopoietic cells R. I. Freshney, I. B. Pragnell and M. G. Freshney (eds.) Wiley-Liss New York Keller, G., Kennedy, M., Papayannopoulou, T. and Wiles, M. V. (1993) Hematopoietic commitment during embryonic stem cell differentiation in culture Mol.Cell.Biol. 13: 473-486 Müller, A. M. and Dzierzak, E. A. (1993) ES cells have only a limited lymphopoietic potential after adoptive transfer into mouse recipients Development 118: 1343-1351 Nakano, T., Kodama, H. and Honjo, T. (1994) Generation of Iymphohematopoietic cells from embryonic cells in culture Science 265: 1098-1101 Robertson, E. J. (1986) Pluripotential stem celllines as a route into the mouse germ line Trends. Genet. 2: 9-13 Schmidt, R. M., Bruyns, E. and Snodgrass, H. R. (1991) Hematopoietic development of embryonic stem cells in vitro: cytokine and receptor expression Genes Develop. 5: 728-740 Spangrude, G. J., Smith, L., Uchida, N., Ikuta, K., Heimfiled, S., Friedman, J. and Weissman, I. L. (1991) Mouse hematopoietic stem cells Blood 78: 1395-1402 Wayne, J., Suh, H, Misulovin, Z, Sokoi, K A, Inaba, K. and Nussenzwig,
M C (1994) A regulatory role for recombinase activating genes RAG-1
and RAG-2 in T cell development Immunity 1 95-107 Wiles, M V (1993) Embryonic stem cell differentiation in vitro
Meth.Enzymol 225900-918 Zeigler, F C., Bennett, B D, Jordan, C. T., Spencer, S D., Baumhueter,
S., Carroll, |