|
Laboratory of Tumor Cell Biology. National Cancer Institute. NIH. Bethesda. Maryland. USA It is a particular honor for me o give the first Frederick Stohlman memorial lecture because of my personal respect and friendship with Fred. His courage. honesty. and stimulation of good science in the hematological field will be remembered by all who knew him. It is also very fitting to have these lectures in Wilsede where special friends were made at the first meetings and where association and friendship with Fred Stohlman became much closer for many of us.
There have been enormous advances in the therapy of lymphocytic malignancies as exemplified by the treatment and apparent cures of some childhood acute lymphoblastic leukemias (see D. Pinkel elsewhere in this book). The myelogenous leukemias remain much more difficult to treat. and for this reason and because they also provide an interesting hematopoietic system for the study of differentiation our interests for the past several years has focused on the origin and pathogenesis of this disease group. The origin of the myelogenous leukemias appears to involve proliferation of astern cell with various degrees of commitment to differentiation and arrests in differentiation at various cellular levels. These diseases appear to be monoclonal (see Fialkow and also Rowley elsewhere in this book) when they are clinically manifest. However. it is not known if the initiation of the disease is multiclonal or monoclonal. Their appearance as monoclonal when presented to clinicians might be because of a select growth advantage of one clone as often seen in tissue culture. We do not yet know the mechanism(s) initiating the abnormality of growth characteristic of these diseases. but certain factors have clearly been shown in select cases to induce or play some role in leukemic development. Studies in mice. chickens. and humans implicate genetic factors which may be involved at multiple levels. Thus. some congenital diseases with chromosomal abnormalities and certain families have shown an unusual incidence of leukemia (see report by R. Miller elsewhere in this book). One family of unusual interest has recently been described by F. Gunz and his colleagues in Australia. Thirteen members of the family developed some form of myeloproliferative disease. clearly implicating genetic factor(s). Yet. some members developed the disease within a relatively short time of each other even though the age of each varied [17]. This certainly suggests an environmental factor may have been operative as well as genetic factor(s). There was no known unusual exposure of any member of the family to chemicals or radiation. What about environmental factors? Radiation can induce leukemias in animals" and it has been clearly associated with myelogenous leukemia in humans under unusual circumstances. Benzene also has been associated with leukemia in very rare instances. and some other chemicals with such rarity that their leukemogenic potential in man is undefined. Epidemiological studies indicate that leukemia is not increased in people living at higher altitudes with greater exposure to radiation" nor is leukemia generally higher in industrial areas than in rural regions [24]. These observations together with the facts that I" the incidence of childhood leukemia has apparently stayed about the same since industrialization and in fact has declined in recent years, 2" the incidence peaks in young children. 3" the incidence is greater in whites than in black people. and 4. association with chemicals and radiation is very rare (see R. Miller elsewhere in this book) all suggest to me that the leukemias may be predominantly biological diseases and that all biological factors must be thoroughly explored.
We have felt that a search for retrovirus (RNA tumor virus" oncornavirus) information or related information in human cells was mandated by several considerations. I. As discussed above epidemiological considerations are a stimulus for consideration of biological factors. Although these ,same broad studies also do not lend strong support to a virus causation of the disease in a conventional manner, I think they are quite consistent with a role for viral information if one considers: a) long latency" b) a second or third factor in addition to appropriate viral information may be a requisite. c) retroviruses can be vertically transmitted either in the germ line as an endogenous cellular element or by congenital infection (see R. Weiss elsewhere in this book). These factors would obscure epidemiological approaches. 2. Several retroviruses can produce leukemia in a variety of animals in laboratory experiments. 3. Retroviruses can sometimes transform cells and not be seen again as discrete virus particles in in vitro experiments (see P. Duesberg elsewhere in this book). Moreover" some data suggests this may also be true with some naturally occurring leukemias of animals. For instance. in a significant number of cats with leukemia (perhaps approaching 50%), feline leukemia virus (FeLV) has not been found (see 0. Jarrett elsewhere in this book). In many of these cats antibodies to FeLV has also not been identified, and recent data from our laboratory in collaboration with W. Hardy and M. Essex indicate that FeLV proviral nucleic acid sequences may also not be readily detectable (Koshy, Wong-Staal" Gallo, Hardy, and Essex" in preparation). Yet there is evidence that FeLV may still cause the disease in these so called "virus negative" cats. This is based on some epidemiological results (M. Essex, personal communication) and on the finding of feline oncornavirus membrane antigen (FOCMA) in the leukemic cells. This protein is believed to he specifically coded for or induced by FeL V and by feline sarcoma virus (FeSV) (see M. Essex elsewhere in this book). 4. Bone marrow transplants studies have indicated that in some exceptional cases normal hone marrow donor cells may be transformed to leukemic cells when given to a leukemic individual [43]. 5. We have recently been able to show that some of the primate type-C retroviruses can transform human Blymphocytes and may also interfere with differentiation of myelopoietic cells, 6, The most compelling reason to think of retroviruses in the etiology of leukemias ofhumans is the results of animal models. It appears now that in every instance where we know the cause of naturally occurring leukemia in a sizable fraction of a leukemic animal population it involves a retrovirus. This now includes chickens (see R. Weiss and 0. Jarrett, A. Burney elsewhere in this book), some wild type mice [16], cows (see 0. Jarrett in this book), cats (see Jarrett and also Essex) and gibbon apes (see next section). 7. Since retroviruses can recomhine with cellular genes and since some of them can affect cell differentiation (see later section of this report and also reports by M, Dexter, by N.Teich, by M, Moore, and by T. Graffin this book), it is possible and perhaps likely that sometimes these viruses contain cell derived genes involved with growth and/or differentiation. If so then it should be important to use retroviruses as probes in human leukemia to see if such genetic information is altered during leukemogenesis whether or not the disease is due to a virus. For this we would choose a primate retrovirus.
There were no isolates of any primate retrovirus before this decade.
Now there are many, and they are from diverse species. I will focus
on two groups since they were the earliest isolates and the only
ones which have been shown to have pathological effects on cells.
we have been particularly interested in the members of the infectious
type-C virus group isolated once from a woolly monkey and called
simian sarcoma virus (SiSV), simian sarcoma associated virus (SiSA
V) complex and some isolated several times f'rom gibbon apes and
collectively called gibbon ape leukemia virus (GaLV). SiSV (SiSAV)
and the various GaLV are very closey related viruses, and the evidence
suggests their ancestral origin Was probably a rodent virus which
entered these primates by interspecies infection [27,47]. It is
of particular interest that the virus entered two primates which
are only distantly related. We have especially focused our attention
on this virus group because the gibbon is the species closest to
man for which a retrovirus has been isolated, because it is the
species closest to man for which we have an animal model of leukemia
and notably one which we know something of the etiology, and because
we think viruses like this have been in humans. It 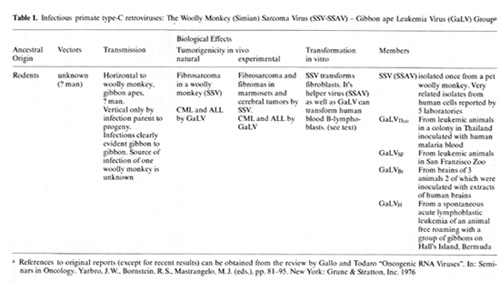
Table 2. Endogenous Type-C Virus or Baboons (BaEV)a 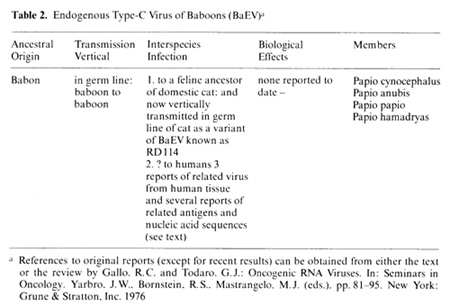
Numerous reports suggest that some fresh uncultured human cells
contain intracytoplasmic virus like particles (discussed in references
[10] and [37]), but since these are not isolated as infectious virus
and have not been shown to exhibit biological activity it is not
certain thay they represent defective or abortive type-C viruses
or are cellular artifacts. However, the presence of high molecular
weight RNA with some sequence homology to RNA from some viruses
and, in some samples of human leukemic cells, a DNA polymerase with
properties resembling reverse transcriptase (RT), associated with
these "particles", emphasize their viral-Iike properties. In some
cases these polymerases were shown to be immunologically specifically
related to RT from SiSV-GaLV group (reviewed in references [10,11]
and [12]), but in most cases immunological relatedness to primate
viruses was not found and extensive comparisons (by immunological
tests) to other animal viruses was usually not performed because
of limited amount of enzyme. These results were a stimulus to consider
the possibility that humans may harbor viruses related to this group.
Our objective was to purify sufficient enzyme to enable us to make
peptide maps of the RT and compare this to RT from these viruses.
This has not been achieved, and there has been little progress in
our laboratory with this problem since the initial reports. On the
other hand Chandra and colleagues [1,2] have reported the purification
of this enzyme (related to SiSV-GaL V RT) from the spleen of a child
with a preleukemic disease, myelofibrosis, which eventually turned
into AML. Similar enzymes have been found in orbital chloromas from
leukemic people living near Ankara, a disease appearing as a cluster
(see R. Miller and also P. Chandra elsewhere in this book). On rare
occasions whole type-C virus has been reported isolated from human
cells. These reports, reviewed in references [II] and [13], have
come from our laboratory (HL23V), from Panem and Kirsten (HEL-12),
from Nooter and Bentvelzen and their colleagues (SKA-21), from Gabelman
and Waxman, and recently from H. Kaplan and colleagues. In each
case the viruses are related to the SiSV-GaL V group, and at least
in three cases (HL23V, SKA-21, and HEL-12), a second virus related
to BaEV has been detected. This peculiar combination remains unexplained.
Because of their similarity to existing primate viruses it is possible
that these isolates are all laboratory contaminants. Regarding HL23V
we offer the following results against contamination: I. Reproducible
isolation from separate clinical specimens: 2. previous evidence
reported in references [30] and [7] for RT related to RT of SiSV-GaLV
in the uncultured blood cells; 3. previous evidence for cytoplasmic
RNA sequences related to SiSV and BaEV in the fresh uncultured blood
cells of this patient [33]; 4. previous evidence for DNA proviral
sequences related to BaEV [33,48], although SiSV proviral sequences
could not be detected [33,48]. We did not subsequently find evidence
for a humoral antibody response to either the SiSV or BaEV component
of HL23V in the sera of this patient, but recently we have obtained
confirmation of the presence of the BaEV provirus in the DNA of
the uncultured blood cells from this patient. As described elsewhere
in detail in this book [49], we have used the technique of DNA digestion
with a restriction endonuclease followed by separation of DNA fragments
by agarose gel electrophoresis, transfer of the DNA fragments to
nitrocellulose filters by the Southern blotting technique, hybridization
of I125-labeled 35S viral RNA to this DNA, and examination for virus
specific bands after development over X-ray films. This approach
allows for more sensitive molec ular hybridization because much
of the irrelevant DNA is excluded (the labeled viral RNA probe is
in excess) and for qualitative assessment because the positive bands
can be visualized and compared to bands of virus infected cells.
As shown elsewhere in this book [49] DNA from fresh uncultured blood
cells from patient HL-23 and from another AML patient labeled HL-49
contain several clearly visible bands after digestion with the endonuclease
HIND III which hybridize to l12.5-35S RNA from BaEV (M7). For control
purposes DNA from normal human leukocytes and from human cells (A204)
deliberately infected by BaEV are also shown. No viral specific
fragments with BaEV as a probe were found in the normal cell DNA.
The only band seen is from ribosomal DNA which was detected with
labeled rRNA which was used as a control because sometimes rRNA
can contaminate viral RNA. Multiple viral specific bands are, or
course, found in the positive control, BaEV infected A204 cells.
It is interesting that some of the proviral bands found in the DNA
from the leukemic cells of patients HL-23 and HL-49 are not found
in the BaEV infected A204 cell positive control. This suggests that
the integration sites may be different or that the viral fragments
detected in HL-23 and HL-49 are related but not identical to the
M7 strain of BaEV. Despite the evidence for some cytoplasmic RNA
sequences related to SiSV (SiSA V) in HL23 fresh blood cells [33]
no novel proviral bands of SiSV (SiSA V) were found. M. Reitz in
our laboratory had previously shown that DNA from the kidney and
spleen of patient HL-23 contained more SiSV (SiSA V) hybridizable
sequences than did DNA from normal cells or DNA from the leukemic
blood cells of patient HL-23. It is possible that only a small percent
of the cells contained the SiSV (SiSA V) proviral sequences, and
failure to detect them is due to insufficient sensitivity. Such
cells may have been concentrated in the kidney and spleen. Unfortunately,
DNA or tissue from these organs is no longer available so they cannot
be examined by the restriction endonuclease blotting technique.
In summary, we have evidence now that the BaEV component of HL23V
was present in the primary uncultured blood cells of patient HL-23,
but inconclusive data for the SiSV (SiSA V) component. The important
question regarding these or similar viruses is to ask whether they
could be important causes of human leukemia. In an effort to obtain
a preliminary answer to this question we surveyed DNA from many
human tissues for the presence of integrated novel viral sequences
by the standard techniq ues of molecular hybridization. We used
molecular probes (both labeled RNA and labeled cDNA) from many animal
viruses, including SiSV and BaEV. We did not find significant differences
between normal and leukemic cells with probes from the majority
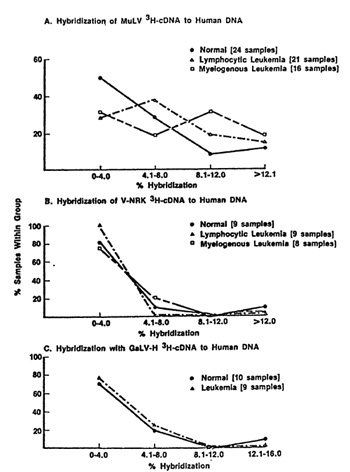
of viruses, and the level of hybridization were insignificant. An
example of this is the negative data obtained with a rat type-C
endogenous virus and with GaLV illustrated in Fig. 1 (parts B and
C). The data is a summary of results of many samples presented as
a distribution frequency, i.e., it represents the percent of human
DNA samples (ordinate) which hybridize to a certain maximum extent
(abscissa). Note that the vast majority of cases (normal and leukemic
hybridize very little to rat virus nucleic acid probes and the distribution
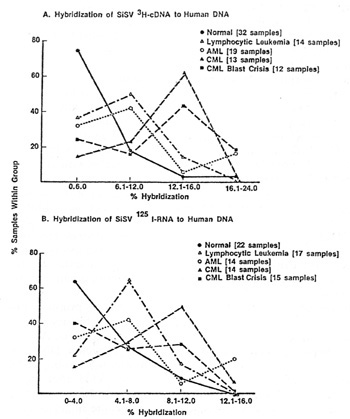
One of the most important questions relevant to type-C viruses and humans i" whether a serological response against them is detectable. Results presented previously [25,38] and elsewhere in this book by R. Kurth and also by H. Snyder and by N. Hogg and their colleagues deal with this complex and as vet unsettled issue. We recently explored a different approach. Stimulated by the fIndings of I. Witz and his colleagues [45], P. Jacquemin, C. Saxinger, and I examined human blood cells for surface immunoglobulins. Both IgG and IgM were found, and appear to be chiefly associated with non-lymphocytic cells, a finding in agreement with Metzgar et al. [29] and Cotropia et al. [5]. We discovered that among the IgG were some which react with high specificity and at low concentrations with purified reversc transcriptases (RT) from select mammalian leukemia typeC viruses. In AML we find the IgG generally reacts with RT from SiSV, and to our surprise the reaction can be specific enough to distinguish this RT from the RT of the other members of the SiSV-GaLV group. In normal people (bone marrow) we find about 20% positive for IgG chiefly reactive with RT from one of the GaLV isolates, namely GaLVsI. In CML we find the remission and chronic phase patients to be like normal (negative or reactive with RT from GaL VsF), while in almost every CML in blast crisis the IgG is chiefly reactive with RT from FeLV and rodent type-C viruses [18,36]. Unlike the sporadic detection of apparent inserted viral nucleic acid sequences the finding of IgG with specific RT enzymatic neutralizing activity is common. Because of this and because we have not as yet dcvcloped an assay independent of RT activity neutralization,l it is premature to conclude that this IgG represents an immune response to a viral protein. It is possible that the IgG is directed against determinants of a protein coincidentally very close to the amino acid sequences of the catalytic sites of certain RTs. The answer to this question should come from the isolation and characterization of the putative cell surface antigen.
We have reported that in the presence of conditioned media (CM) from certain human embryo cell strains, human myelogenous leukemic cells can often be induced to grow in liquid suspension tissue culture [8,14]. In many instances the cells terminally differentiate [14,15]. Sometimes they retain marker chromosomes, and this observation combined with the fact that this CM generally does not induce growth of normal myeloid cells led us to conclude that the maturation arrest of many human myelogenous leukemias is not always irreversible, in agreement with L, Sachs [35] and M. Moore [31] and their colleagues. The differences in response of normal myeloid cells and leukemic cells also suggested to us that membrane receptors for regulation may be modified in leukemia. An interesting by product of this research was the establishment of the first human cell line with distinct myeloid characteristics [3]. This line, called HL-60, was shown to consist mostly of promyelocytes [31] which can be terminally differentiated when DMSO or other compounds active in the Friend murine erythroleukemia system are added to the culture [3].
To our knowledge there are surprisingly no reported studies of
the effects of primate type-C viruses on fresh human blood cells.
We have recently completed a preliminary study of the effects of
various type-C viruses, including the primate viruses described
here on human blood and bone marrow cells. We find that SiSV (SiSV),
SiSA V alone, GaLV, HL23V (SiSV component), but not BaEV induce
growth (Fig. 3) of B-Iymphocytes (Fig. 4). In about one third of
cases studied, the cells become immortalized, and despite apparent
normal karyotype, they are tumorigenic in nude mice (Fig. 5). These
cells are Ig and EBNA positive and do not have myeloid or T-cell
characteristics [28]. The observation is different from that of
"spontaneous" transformation of EBV positive human B-Iymphocytes
because the induction of growth is faster, the frequency of establishment
of cell lines in our hands much greater, and the cells are tumorigenic
despite apparent normal chromosomal makeup. The addition of BaEV
or FeLV (despite some virus replication in a few cases) did not
produce this effect. Finally, recent results indicate that the same
phenomenon can be induced with EBV negative cord blood but at a
lower frequency. We conclude that by indirect or direct means and
by mechanisms not yet understood, some primate type-C viruses can
be involved in the in vitro transformation of human B-Iyrnphocytes.
A currently interpretable and simple model for leukemogenesis is
that the leukemogenic event whatever the cause, leads to auto-production
of a growth promoting molecule which prevent binding of normal regulatory
molecules. This model is similar to one proposed by Todaro and colleagues
in the genesis of sarcomas [6]. The alternative is that receptors
for regulators are themselves modified. These ideas should become
testable in the very near future. 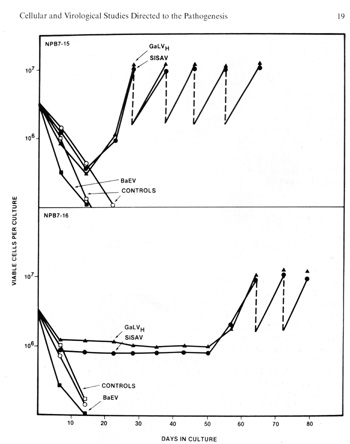 Fig.3. induction of growth of B-Iymphocytes from normal human blood by primate type-C retroviruses (Gal" VH and SiSA V). Human blood leukocytes were cultured with or without the Hall's Island strain of GaLV or with the simian sarcoma associated virus (helper virus) (SiSAV). In some cases (e.g.. sample NPB 7-i5 shown in the figure) induction of growth was rapid In other cases (e.g., sample NPB 7-16 shown in the figure lower panel) growth induction occurred after 50 days Spontaneous growth (no virus) occurred in only 1 to 2% of samples but in more than 40% of cells treated with viru5 if a co-cultivation method was used (P Markham. F. Rucetti. Z. Salahuddin. R Gallagher, and R. Gallo. in press). 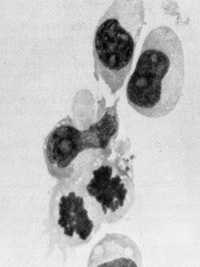 Fig. 4. Morphology of „immortalized cell line induced from normal human blood after infection by Ganot IVH and EBV positive ( Magnification 1000 x ) 
1. Work over the past years and especially results of the past
few years indicate that type-C viral or viral related genetic information
exists in humans.
I. Chandra. P.. Steel. L. K.. Purification. biochemical characterization
and serological analysis of cellular deoxyribonucleic acid polymerases
and a reverse transcriptase from spleen of a patient with myelofibrotic
syndromc Biochem l 167, 513-524 (1977) |