|
Cancer Research Unit, Walter and Eliza Hall Institute,
P.O. Royal Melbourne Hospital, 3050, Victoria, Australia
Understanding of the abnormal nature of cancer cells has advanced
rapidly in the past decade because of work in two apparently separate
fields those of oncogenes and specific growth-regulatory factors.
What has intrigued workers in both fields has been the recognition
that, in a number of instances, the products of oncogenes or proto-oncogenes
have been shown to be related either to growth factors themselves
or to the receptors for such growth factors. The reported examples
of this association are already numerous enough to make a chance
association improbable c-sis and PDGF [1], c-erb-B and the EGF receptor
[2], c-fms and the CSF -1 receptor [3]. When this association is
considered in the light of the numerous documented examples, particularly
in the leukemias and lymphomas, of non-random chromosomal translocations
that involve proto-oncogenes [4], a strong case exists for formalizing
earlier notions of cancer into a concept that neoplastic change
results from aberrant or aberrantly expressed genes that code for
growth factors or growth factor receptors. Where this concept of
cancer becomes less than adequate is in its extension to two more
specific proposals: (a) that cancer is the simple consequence of
over-stimulation (either excessive or inappropriate persistence)
of the proliferation of the cells involved, or (b) that this over-stimulation
has an origin within the cell itself the autocrine hypothesis of
cancer. At first sight, these extensions seem reasonable, both on
grounds of simplicity and because experimental cancers can often
be transplanted to syngeneic recipients using a single cancer cell,
with the resulting transplanted cancer being documentable as being
derived from the transplanted cell. There are three types of evidence
indicating that these more extreme views of cancer formation are
likely to be naive and incorrect:
Analysis of the factors controlling the proliferation of murine granulocyte-llacrophage populations has shown that the glycoprotein colony-stillulating factors (CSFs) are the only known molecules in biological materials that are able by direct action to stimulate cell proliferation in these populations. Four such murine CSFs have been identified, purified and sequenced, and cDNAs for three have been isolated and expressed in mammalian and bacterial expression systems (Table 1 ). Several features of these CSFs have become evident: (a) All are glycoproteins, but the extensive carbohydrate portion of each molecule seems not to be needed for the biological actions of the molecule on responding cells either in vitro or in vivo. (b) Three are monomers with mandatory disulfide bridges, while one (MCSF) is a diller, also with some form ofnecessary disulfide bridging. (c) No sequence homology exists between the CSFs or between the CSFs and known oncogene products or growth factors for other tissues. ( d) Each CSF has a corresponding specific membrane receptor, one of which (for MCSF) is, or is closely related to, the c-fms proto-oncogene product [3]. (e) Responding granulocyte-llacrophage progenitor cells simultaneously co-express receptors for more than one CSF and cross-down modulation of these receptors can occur following the occupancy of one type of receptor by its specific CSF [13]. Table I. The granulocyte-macrophage
colony-stimulating factors 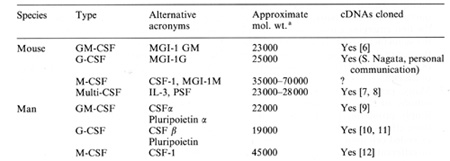
Murine myeloid leukemic cells from primary tumors or recently isolated cell lines display a similar dependency on CSF for proliferation to that of normal cells, although on continued culture in vitro, such cell lines eventually lose most or all of their CSF dependency. Conversely, CSFs can inhibit the proliferation of leukemic cells. In one extensively studied murine myeloid leukemia (WEHI3B D+) the commitment action of the CSFs was demonstrated to have profound effects on the behavior of the leukemic population. Culture of these cells in vitro in the presence of M-CSF (or multi-CSF) did not influence their pattern of differentiation or proliferation [16]. However, culture in the presence of purified GM-CSF or G-CSF led to the production of differentiating granulocytic and monocytic cells [17, 18]. Of the latter two, the action of G-CSF was by far the more striking, and culture for two to six cycles in the presence of G-CSF led to suppression of stem cell self-generation, with complete extinction of these leukemic cell populations in vitro and demonstrable loss of leukemogenicity on transplantation [19, 16]. Single-cell analysis of this process indicated that the irreversible commitment process closely resembled the commitment action of the CSFs on normal granulocytcmacrophage progenitors [20]. A differentiation-unresponsive subline of WEHI-3B cells (D- cells) was shown to be unrespon sive because of failure to express membrane receptors for G-CSF [21 ], although other abnormalities could have co-existed in such cells, preventing their responsiveness. It is of interest that even with responsive but autonomously growing WEHI-3H D+ cells, culture in the presence of G-CSF did result in initial growth stimulation of the leukemic cells [20], suppression of the population commencing only following the induction of significant levels of differentiation commitment. Thus, even in this otherwise optimal system for demonstrating CSF-induced suppression, the opposing actions of the CSFs were clearly evident. This work with WEHI-3B cells has been criticized as dealing only with a single cell line and therefore possibly not being of general relevance for other myeloid leukemias. However, in current experiments a similar differentiation induction has been observed with other murine myeloid or myelomonocy tic leukemias, suggesting that the effects of G-CSF are not restricted to this one model system.
Three sets of observations have recently been reported on the question of whether autocrine production of CSF by emerging leukemic cells is necessary for leukemic transformation. Two groups have noted that when continuous hemopoietic cell lines that are not leukemogenic by transplantation tests subsequently acquire the ability to produce transplanted leukemias, this is associated with the production of detectable amounts of CSF [22, 23]. The possibility raised by these observations is that autocrine production of CSF, by providing an internal, nonregulatable source of the appropriate growth factor, is the final step in the multistage leukemogenic process. A somewhat similar conclusion can be reached from experiments in which cells of the non-Ieukemic FD-CPl cell line were transformed to leukemogenic cells by the insertion of a retroviral construct containing GM-CSF cDNA [24]. The resulting constitutive production of GM-CSF on which the FD cells are dependent for survival and proliferation was the only obvious effect of the experimental procedure that resulted in leukemic transformation. A quite different conclusion was reached from studies in which continuous hemopoietic cell lines were transformed to autonomous, leukemogenic cells by infection with the Abelson virus [25, 26]. In this case, no transcription ofmRNA for either GM-CSF or multi-CSF was detected, nor was there detectable synthesis of either CSF or the occurrence of abnormal expression of membrane receptors for either CSF. It is evident from these latter experiments that leukemogenic transformation does not of necessity require either the autocrine production of growth factors or the expression of abnormal numbers of growth factor receptors. Although FD-CPl cells are non-leukemic and absolutely CSF dependent, they are highly abnormal, being immortalized, incapable of differentiation, possessing eight metacentric marker chromosomes, and exhibiting a very high capacity (greater than 90%) for clonogenic self-renewal. The high self-renewal capacity of FD-CPl cells (Table 2) despite their inability to produce transplanted tumors suggests that high selfrenewal is necessary, but not in itself sufficient, for a cell to behave as a leukemic cell. Because of their properties, FD-CPl cells could quite properly be regarded as already having passed through one or more preleukemic changes, so none of the above observations really addresses in totality the question Table 2. Self-generation by clonogenic
FD-CP1 cells compared with self-generation 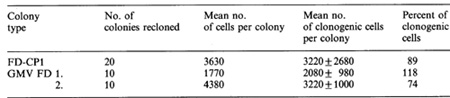
While the CSFs are the only known proliferative agents for granulocyte-macrophage cells, it is important to recognize that CSFs are not the only biological agents able to induce differentiation in myeloid leukemia cells with subsequent loss of leukemogenicity. Extensive studies have documented the presence in various conditioned media of differentiation-inducing factors (OF, DIF or MGI-2) that do not appear to be proliferative agents but are able to induce similar suppressive effects to G-CSF on a variety of murine and human leukemic cell lines, the most extensively studied being the murine M1 model [31-33]. In vivo evidence indicates that production of the murine factor can be T -cell-dependent [34], unlike the situation with G-CSF, and biochemical purification studies have indicated that OF is separable and distinct from G-CSF [33, 35]. Injection of crude material containing MGI-2 inhibited the growth of transplanted myeloid leukemic cells in SL and SJ L/ J mice [36], and it will be of interest to determine the structure and actions of this factor in vivo when cDNA clones and recombinant material can be obtained. Certainly, if DF or related factors lack proliferative effects on responding leukemic cells but exhibit strong differentiating effects, such factors would be quite clearly superior to the CSFs as antileukemic agents. It is quite conceivable that the production of molecules such as DF (MGI-2) could be elicited within a cell in response to CSF signalling and account for the observed differentiation occurring in both normal and leukemic cells following exposure to G-CSF [37, 38]. Any explanation of the differentiation-inducing action of CSFs on leukemic cells requires the production of some type of signalling molecule to mediate the observed effects, and such a molecule would need to achieve an irreversible alteration in the genome of the cell, since the effects are known to be irreversible following CSF removal. What seems improbable is that the DF would need to be secreted by the responding cells and to activate the cells by binding to membrane receptors. This proposal encounters the same types of difficulties as the autocrine hypothesis of leukemia with respect to the CSFs, namely that DF and the CSFs are also produced by other tissues. Significant actions of these molecules might be achieved by remaining within the cell producing them, but the minute amounts secreted by individual leukemic cells are unlikely to be of significance in the context of cells residing in fluid containing the same molecules produced by vastly more numerous cells in other tissues. It is also improbable that secreted DF could be responsible for the differentiation that can be induced by G-CSF in cultures of a single normal cell or leukemic cell in 1-ml volumes, since it is unlikely that anyone cell could synthesize sufficiently high concentrations to achieve any significant binding to membrane receptors. Thus, even though differentiating normal and some leukemic cells have been shown to generate DF or comparable material able to induce differentiation in other leukemic cells [37, 39], the single-cell experiments suggest that if these molecules are critical in differentiation induction, they are likely to act while still within the responding cell and could equally well be regarded as mediator molecules of CSF-induced events.
Turning to the situation with human myeloid leukemia, the most striking observation has been that the clonogenic proliferation in vitro of cells from most patients with both chronic and acute myeloid leukemia is absolutely dependent on stimulation by extrinsically added CSF or by CSF produced by other cells in the culture [15]. More recently, some examples of apparently autonomous growth by acute myeloid leukemia cells have been encountered (C. G. Begley, D. Metcalf, N. A. Nicola: unpublished data), but these are a minority of cases and the CSF dependency of these cells earlier in their evolution cannot be determined. For the large majority of primary human leukemias, therefore, the clonogenic GM cells exhibit no capacity for autonomous proliferation in dispersed suspension cultures, a situation which appears to effectively eliminate an autocrine basis for their neoplastic behavior. The differentiating cells (monocytes) in many populations of CML, AMML and AMonoL have a clear capacity to produce CSF, but this capacity is no higher than that of corresponding normal cells and it occurs in vivo in the context of widespread tissue production of significant concentrations of CSF [40]. From the viewpoint of leukemia development, the above in vitro data would indicate that the CSFs must playa mandatory role in myeloid leukemia development in most patients since the leukemia clone is CSF dependent. However, based on the general comments made earlier, CSF stimulation cannot be the sole leukemogenic event, since this fails to account for the clonal nature of the disease or for the abnormal pattern of selfgeneration following CSF stimulation. These latter two facts require the presence of intrinsic abnormalities in the initiating cell, quite possibly involving the signalling events following CSF stimulation. Different AML populations vary in their pattern of expression of CSF receptors and in their quantitative responsiveness to CSF stimulation. However, in neither case is the variation in these phenotypic characteristics outside the wide range observable in normal progenitors, and such differences between myeloid leukemic populations can be expected since each is clonally derived from a heterogeneous population. For the two human CSFs (GM-CSF and G-CSF) with proliferative effects on human cells the responsiveness of individual AML populations is quite similar, and there has been no evidence that a particular AML population might be uniquely responsive to only a single CSf. To date, leukemic populations from all patients examined with acute and chronic myeloid leukemia have exhibited G-CSF receptors and no examples of the WEHI-3B o- situation have been encountered [14]. Suppression of clonogenic self-renewal in cultures of primary human myeloid leukemia has been difficult to monitor since the clones in conventional CSF-stimulated cultures uniformly lack clonogenic cells. This may indicate an extremely strong commitment action of the CSFs, or it may merely indicate that the clonogenic cells grown in such cultures are already committed and have lost their self-generative capacity. Studies using an alternate culture system for AML blast cells do indicate a capacity for self-generation by clonogenic cells [41]. While this process remains CSF dependent, the regulation of the behavior of true leukemic stem cells in AML populations has not yet been fully characterized [42]. Established human leukemic cell lines can be subjected to the same types of study outlined above for WEHI-3B cells. Like the mouse cell lines, the human HL60 cell line has now become autonomous with respect to dependency on extrinsic CSF, but it remains capable of chemically induced differentiation [43] and can therefore be used to determine the ability ofhuman CSFs also to induce differentiation commitment. The behavior of HL60 cells in clonal culture appears to have been variable in different laboratories, either because of subclone differences or because the fetal calf serum used in such cultures seems to have an influence on the observed effects. Culture in the presence of either GM-CSF or G-CSF does not induce obvious morphological differentiation in these cells but does lead to expression on the membranes of lineage-specific markers associated with maturing cells [44; C. G. Begley, D. Metcalf, N. A. Nicola: unpublished data]. Of more importance, both GM-CSF and G-CSF have an ability to suppress clonogenic self-renewal as assessed by recloning of treated HL60 cells (Table 3). This raises the interesting possibility that suppression of self -renewal need not be accompanied by morphological differentiation and that use of the latter criterion may lead to a serious underestimation of the ability of the CSFs to induce differentiation. Given that the proliferation of human myeloid leukemic cells is usually CSF dependent but that the CSFs can exhibit a capacity to extinguish such a population by differentiation commitment, a dilemma is presented in assessing whether their use would represent a useful procedure in the treatment of myeloid leukemia. Use of the CSFs to accelerate the regeneration of surviving normal clones during remission presents less of a problem unless significant numbers of clonogenic leukemic cells persist during such a remission. These questions have some immediacy, since mass-produced recombinant human GM-CSF and G-CSF will shortly be available Table 3. Action of GM-CSF and G-CSF
on differentiation and clonogenic content of HL60 cells 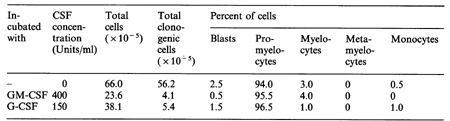
Most primary myeloid leukemias are dependent for proliferative stimulation on the glycoprotein colony-stimulating factors. These agents are therefore mandatory co-factors in the development of myeloid leukemia. The CSFs also modify oncogene transcription, and in model leukemogenesis experiments GM-CSF has been shown to be a proto-oncogene. However, most evidence is against an autocrine hypothesis of myeloid leukemia based solely on CSF production by emerging leukemic cells. Because the CSFs also have differentiation commitment actions, they can induce differentiation in myeloid leukemic cells, and G-CSF in particular has an impressive capacity to suppress myeloid leukemic populations by this action. The antagonistic actions of the CSFs on myeloid leukemic cells make it difficult to predict whether they will prove to be useful agents in the management of myeloid leukemias.
1. Johnson A, Heldin C-H, Wasteson A, Westermark B, Deuel TF, Huang
JS, Seeburg PH, Gray A, Ullrich A, Scrace a, Stroobant P, Waterfield
MO (1984) The c-sis gene encodes a precursor of the B chain of plateletderived
growth factor. EMBO J 3:921-928 |